
13 full members with expertise across the entire pharmaceutical supply chain.

Almac's Clinical Services business unit has expanded its service offering for dispensing and bottling solid dosage products.

The precautionary recall follows manufacturing deficiencies identified at the site in India.

Vetter has ready-to-submit documentation for this service in Common Technical Document (CTD) formats for the US, Europe and Japan.

Molecular Profiles has opened a new facility in the UK following official approval from MHRA.

The new ink takes less a second to dry and is four times more fade resistant than inks that are typically used in retail packaging.

The facility, which includes state-of-the-art formulation, analytical and synthetic laboratories as well as a customer training center, will focus on bioavailability enhancement and oral dosage formulations.

A roundup of developments in global health and sustainability from the bio/pharmaceutical industry, its suppliers, and other public and private organizations.

The new unit aligns existing modified-release and medication-delivery businesses of the company.

The acquisition of Melbourn extends Intertek's offering to include formulation, product development and characterisation of orally inhaled and intranasal products amongst others.

Report outlines recommended practices for control and evaluation of operations.

Research will focus on cancer and viral disease targets not addressable using antibody-based technologies.

Parexo Labs, a division of Azaya, launches as a new CDMO with an emphasis on nanotechnology and liposomal manufacturing.

Resource will foster innovation in DEM simulation for process industries.

Novartis and Biological E, a biopharmaceutical company based in India, have entered into an agreement that aims to deliver affordable and accessible vaccines for typhoid and paratyphoid A fevers to developing countries and thereby address the unmet medical need in endemic regions.

The core DNA-sequencing technology is based on the concept of passing a single strand of DNA through a nanometer-scale pore and reading out the sequence directly as it does so.

Almac introduces handling and bottling capabilities in EU and US headquarters.

Seattle Genetics could potentially receive approximately $500 million in fees, milestones and royalties.

CPhI is now accepting applications for the 2013 CPhI Pharma Awards, open to individuals and companies who have developed innovations in pharma over the past year.

Europe prepares for inclusion of Croatia in EMA activities.

The move doubles the CRO’s capacity in Europe.

The partnership provides access to Xencor’s Fc engineering patents for monoclonal antibodies.

Programs assist in the fast track of drugs for serious conditions.

Companies hope the collaboration will result in identifying potential new drug candidates using fewer rounds of design, synthesis, and testing.

The companies agree to jointly develop and promote MOR202, an oncology mAb in a deal potentially valued at more than $800 million.

The Onyx board of directors rejects $120-per-share proposal as significantly undervaluing the company.

Brazil offers opportunities and challenges for global pharmaceutical companies.

Falsified Medicines Directive requires imported APIs to have written confirmation of GMP standards.

The $1-billion deal includes select parts of Merck?s manufacturing site in Oss, The Netherlands and Sioux City, Iowa as well as 11 finished products.
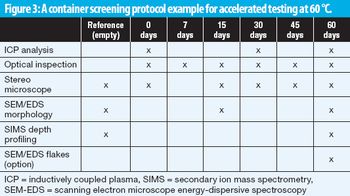
A screening method aligned with USP 1660 guidance predicts glass delamination in primary packaging for parenterals.