
A federal judge entered an order of permanent injunction against Paul W. Franck, the owner and operator of several compounding pharmacies in Florida.

A federal judge entered an order of permanent injunction against Paul W. Franck, the owner and operator of several compounding pharmacies in Florida.

The companies have agreed to an all-stock merger of equals transaction anticipated to close in the second half of 2016.

The collaborations have the goal of creating new tools that will help predict how people with Type 2 diabetes adhere to their medication.
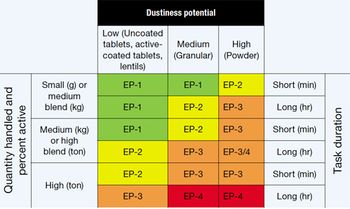
Safe handling of HPAPIs requires determining exposure potential and selecting appro-priate containment strategies.

Experts discuss some of the emerging trends in bioprocessing in 2016, including 4D bioprinting, 2D-NMR, and the CAR-T design space.

Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses how to report quality metrics to FDA.

Policies for patient access to life-saving therapies must keep pace with biomedical innovation.

MilliporeSigma, the North American life-sciences business of Germany’s Merck KGaA, added a 2000-L single-use bioreactor to its facility in Massachusetts.

EMD Serono, the North American biopharmaceutical business of Germany’s Merck KGaA, will expand its R&D facility in Massachusetts, US.

Pharma understands the importance of patient centricity, but the problem is the gap between intent and action.

The agency extends the indication of the drug in combination with bendamustine.

The agency recommends the approval of Zavicefta to treat infections caused by resistant bacteria.

Hacene Mekerri will serve as vice-president of PPD’s central laboratory organization.

Air Cargo Community Frankfurt takes steps to make Frankfurt Europe’s largest certified pharmaceutical hub.

Quotient Clinical has expanded its clinical spray drying capability through the acquisition of a Niro Mobile Minor Spray Dryer.

Worthington’s CryoScience by Taylor Wharton business will design and manufacture biostorage and logistics equipment for use in Cryoport’s life-sciences cryogenic logistics solutions.

Platinum Press provides serialization-ready printed materials to apply final track-and-trace data onto packaged pharmaceutical products.

Almac partnered with Optel Vision to provide a line-level solution (hardware and software) integrated with Almac’s proprietary level three, site-level software.

Immuno-oncology drugs are demonstrating patient benefits, but growing resistance to the high cost has implications for patients, market access, and manufacturers.

The agency holds a workshop to strengthen collaboration with healthcare providers.

MSD joined the WHO campaign in Africa, which is focused on staying polio free.

AbbVie will acquire Stemcentrx and its lead late-stage asset rovalpituzumab tesirine (Rova-T), a novel biomarker-specific therapy derived from cancer stem cells.

The company voluntarily recalls product due to particulate matter.

The company’s preservative-free multidose eyedropper wins award.

The company announced the launch of its first-in-class Lynx CDR connectors at INTERPHEX 2016.

The VeriPac UBV Leak Detection System is designed specifically for multi-cavity blister packs.

The ink has a fast dry time, making it suitable for pharmaceutical packaging and labeling.

The agency publishes draft guidance on assay development and validation for immunogenicity testing.

WHO report highlights goal of eliminating malaria in 35 countries by 2030.