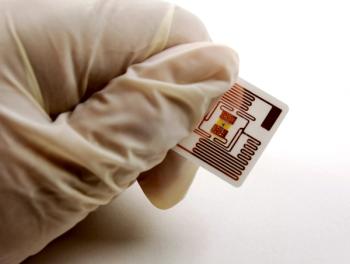
The new label for prefilled syringes, in conjunction with innovations from SCHOTT Pharma, enables integrated tamper-evidence and digital tracking across the supply chain.

The new label for prefilled syringes, in conjunction with innovations from SCHOTT Pharma, enables integrated tamper-evidence and digital tracking across the supply chain.

At the Cell and Gene Meeting on the Mesa, a panel discussion was held on advanced therapy production and how it demands modular platforms, automation, and data governance to drastically improve patient access and affordability.

The awarded $1.7 million in funding will support the company’s preclinical and clinical progress on DMX-1001 (noribogaine), designed to reduce relapse and improve brain health in patients with alcohol use disorder.
The growing demand for liquid medicines is increasingly driven by the unique needs of several distinct patient groups, improving compliance in these demographics through flexible, palatable dosing options.

The licensing of Talicia, an FDA-approved fixed-dose therapy, to key at-risk markets strategically combats an increasingly high antibiotic resistance to the cancer-causing bacteria.
Pharmaceutical Technology® spoke with Juliana Maynard, PhD, Head of Translational Imaging at Medicines Discovery Catapult, to find out what makes radiopharmaceuticals unique and how MDC’s collaboration with Crown Bioscience can help developers of these treatments for cancer.

Artificial intelligence and machine learning are anticipated to boost success rates.

Artificial intelligence, machine learning, and novel instruments are helping drug developers evaluate complex data from bioanalytical studies.

In the conclusion of this three-part series, the author explores the potential use of agentic AI in pharmaceutical R&D.

Siegfried Schmitt, PhD, vice-president, Technical at Parexel, answers questions on the use and benefits of real-world evidence for small-molecule and large-molecule drug development.

Pharma news on AI's role in drug development, new FDA/EMA guidance, clinical advances, MFN pricing agreements, and ongoing quality control challenges.

Pharmaceutical Technology® spoke with Juliana Maynard, PhD, Head of Translational Imaging, at Medicines Discovery Catapult, to find out what makes radiopharmaceuticals unique and how MDC’s collaboration with Crown Bioscience can help developers of these treatments for cancer.

Pfizer re-balances weight loss portfolio with Metsera, Bristol Myers Squibb and Roche make breast cancer progress, and Keytruda gets under your skin.

This article takes a look at the developing use of AI in pharmaceutical development and manufacturing.

The go-ahead for Novartis’ oral treatment marks a new option for patients with spontaneous urticaria who remain symptomatic despite antihistamine therapy.

This review of the nitrosamines contamination problem in pharmaceuticals takes a look at how the crisis started and developed.

Sept. 30 marked 61 days since July 31, one day past the timeframe President Donald Trump had set forth for companies to lower prescription drug prices in the United States.

A draft reflection paper on patient experience data is up for public consultation until Jan. 31, 2026.

FDA's Center for Biologics Evaluation and Research has released updated draft recommendations for sponsors of cell therapies, gene therapies, and tissue products.

In this continuation of a three-part series, the author explores the potential use of agentic AI in pharmaceutical R&D.
Pharmaceutical Technology® spoke with Bryan Miller, Director of Scientific and Technical Operations at Crown Bioscience UK, to learn more about Crown Bioscience’s collaboration with Medicines Discovery Catapult on a new development platform for radiopharmaceuticals.

Your weekly news hub for biopharma trends, FDA regulations, M&A deals, supply chain insights, drug development innovations from BIO-Europe, and more.

The 100% tariff on imported drugs will pressure pharma companies to build manufacturing sites in the US or face significant costs.

In this episode of the Ask the Expert video series, Susan J. Schniepp, Regulatory Compliance Associates, and Siegfried Schmitt, PhD, Parexel, answer questions on the use of real-world evidence for both small-molecule and large-molecule drug development. In addition, they tackle a question on supply chain security problems that arise during transportation of pharmaceutical goods.

Vienna to host BIO-Europe 2025, connecting top biopharma companies for collaborations and pipeline development.

The FDA draft guidance "Considerations for Complying with 21 CFR 211.110" raises points to consider regarding drug products made using advanced manufacturing, batch uniformity, drug product integrity, and how manufacturers can incorporate process models into control strategies.
Alvin Jogasuria, ProBio; Matthew Lunning, University of Nebraska Medical Center; and Carl Schoellhammer, DeciBio, go behind the headlines to discuss the need for doing more with less.

The dispute shines a light on the vulnerability of long-established drugs to renewed safety scrutiny: Even when causal evidence is lacking, observational findings can influence policy and public perception.
Pfizer gains access to Metsera’s oral and injectable obesity candidates, underscoring innovation in drug development and scalable manufacturing.

Advances in digital technologies offer effective data handling for bio/pharma manufacturing.