
The proposed $12.7-billion deal includes the spinoff of GSK and Pfizer consumer brands to a new UK-listed company.

The proposed $12.7-billion deal includes the spinoff of GSK and Pfizer consumer brands to a new UK-listed company.

The STERIS ProKlenz ONE alkaline cleaner now has a label performance claim for biofilm removal, in addition to the product’s existing label as a disinfectant and virucide.

Agilent and other partners are funding development of Tapestri, a single-cell sequencing platform designed to help predict cancer relapse in individual patients and show the efficacy of gene-editing experiments.

The use of artificial intelligence creates growth opportunities in novel therapeutics development by leveraging multi-sourced data, according to experts at research and consulting firm Frost & Sullivan.

The company’s biosimiliar to Roche’s Avastin (bevacizumab) received a positive opinion for marketing authorization from the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP).

The agency sent a warning letter to Cao Medical Equipment Co., Ltd. after inspectors found CGMP violations at the company’s Langfang, Hebei facility.

The companies will develop a digital platform for the development and lifecycle management of antibiotics.

Asclemed USA Inc., dba Enovachem Pharmaceuticals is recalling the product due to labeling that incorrectly states that stoppers do not contain latex.

Roquette has announced the completion of its acquisition of a majority stake in a pharma packaging company based in India.

The UK Pharma Industry has emphasised the need for the government to avoid a 'no-deal' Brexit in response to the prime minister delaying the deal vote.

Nanoform has unveiled its plans to construct a GMP manufacturing plant that will provide API ‘solution to particle’ nanoization for clinical trials

Eli Lilly and AC Immune have signed a license and collaboration agreement focussing on the R&D of tau aggregation inhibitor small molecules for the potential treatment of neurodegenerative diseases.

According to research from staffing firm, Kelly Services, 14% of life science professionals based in the UK may move abroad if Brexit results in negative changes to economic conditions.

Avacta Group and LC Chem Life Sciences have entered into a multi-target collaboration and development agreement.

Capital Recovery Group, Federal Equipment Company, Heritage Global Partners, and PPL Group purchased a 110-acre campus with a flexible pharmaceutical manufacturing and packaging facility from a generics manufacturer in Huntsville, AL.

The merged companies will provide histopathology services in the research and pre-clinical therapeutics markets.

A new facility in California will expand Orchard Therapeutic’s capacity to develop and deliver lentiviral vector and gene-corrected hematopoetic stem cells.

FDA has withdrawn the proposed rule that would have allowed generic-drug makers to independently update and distribute new safety information in drug labels.

The $62-billion acquisition of Shire by Takeda Pharmaceutical was approved by both sets of shareholders.

New FDA guidance developed to identify lapses in data integrity and promote best practices.

FDA cites Zhejiang Huahai Pharmaceutical in valsartan impurity investigation.

TrendMiner 2018.R2 software for analysis of time-series data includes the ContextHub platform for expanded, self-service analytics capabilities.

AqVida, winner of the 2018 CPhI Excellence in Pharma Award for manufacturing technology and equipment, identifies advantages of robotics compared to conventional automation in fill/finish operations.

The SafeGuard Preventative Maintenance Solution from PSG’s Griswold provides remote monitoring for a centrifugal pump and its motor.
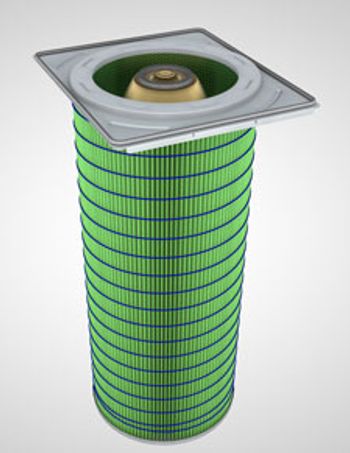
The Gold Cone X-Flo Filter Cartridge from Camfil APC stays cleaner and lasts longer than conventional pleated filters.

The company is investing approximately $14 million to expand biologics packaging capabilities and capacity at its biologics manufacturing facility in Bloomington, IN.

The company will use GE Healthcare’s off-the-shelf KUBio biologics factory, which is expected to start operations in 2020, to provide development and manufacturing for early- to late-clinical and early-commercial manufacturing stages.

FDA has approved Truxima (rituximab-abbs), a biosimilar to Roche’s anti-cancer biologic, Rituxan (rituximab).

The companies aim to advance research into inflammatory bowel disease.

GSK and TESARO have entered into a definitive agreement concerning GSK's acquisition of TESARO for an aggregate cash consideration worth approximately $5.1 billion.