PTSM: Pharmaceutical Technology Sourcing and Management
In the ongoing search for better fluorinating reagents, solid reagents and new coupling reactions are showing promise.

PTSM: Pharmaceutical Technology Sourcing and Management
In the ongoing search for better fluorinating reagents, solid reagents and new coupling reactions are showing promise.
PTSM: Pharmaceutical Technology Sourcing and Management
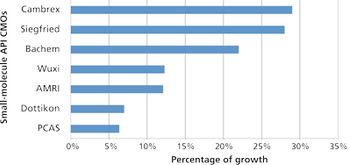
While biologics development grabs investor interest, small-molecule APIs still hold two-thirds of the drug-development pipeline.

PTSM: Pharmaceutical Technology Sourcing and Management
The Transform hydrate-able film from Lubrizol LifeSciences is compatible with a range of APIs.

PTSM: Pharmaceutical Technology Sourcing and Management
Citing compliance issues, FDA extends deadline for product tracing information requirements for dispensers to March 1, 2016.

PTSM: Pharmaceutical Technology Sourcing and Management
Ashland presented a science-based strategy to enhance pharmaceutical formulation quality and advance drug delivery at AAPS 2015.

PTSM: Pharmaceutical Technology Sourcing and Management
CDMO Xcelience opens new facility in Tampa, FL, for formulation and analytical services.

PTSM: Pharmaceutical Technology Sourcing and Management
FDA’s inactive ingredients database (IID) has been under development for several years, as a way to allow generic and name-brand pharmaceutical manufacturers to check on the safety and performance of specific inactive ingredients.

PTSM: Pharmaceutical Technology Sourcing and Management
Catalent Pharma Solutions announced plans to quadruple the cold-chain capacity at its existing clinical supply storage and distribution facility in Shanghai, China. The expansion comes in response to increasing demand from domestic and multinational pharmaceutical sponsors and contract research organizations (CROs).

PTSM: Pharmaceutical Technology Sourcing and Management
Croda Chocques SAS' excipient facility in France has received EXCiPACT certification from SGS ICS, making it the 24th manufacturing site to be certified to the scheme, which verifies that manufacturing of pharmaceutical-grade excipients meets current good manufacturing practices (cGMPs). It is the third Croda site in Europe to receive this certification in the past year. Certification reflects a rigorous assessment program, both for the auditor and the audited, and the auditor's report had to be verified by an independent certification board.

PTSM: Pharmaceutical Technology Sourcing and Management
Capsugel has launched its enTRinsic drug-delivery technology platform. Using pharmaceutically approved enteric polymers, the technology offers full enteric protection without the need for functional coatings and enables targeted release of gastric acid- and heat-sensitive active ingredients in the upper gastrointestinal tract. The new technology platform expands Capsugel’s range of modified- and targeted-release solutions and biotherapeutic formulation offering, and can be used for oral delivery of drugs, including vaccines, proteins, and peptides.

PTSM: Pharmaceutical Technology Sourcing and Management
Rx-360 announced that Mark S. Paxton has been appointed as the new chief executive office of the international pharmaceutical supply chain consortium. This appointment follows the successful growth of Rx-360 since its foundation in 2009 in the aftermath of the adulterated heparin tragedy. Rx-360 has 300 volunteers from more than 100 institutions that are organized in 17 working groups with activities in the US, Europe, China, and India.

PTSM: Pharmaceutical Technology Sourcing and Management
EMD Millipore, the life-science division of Merck KGaA, has introduced Parteck SRP 80, a new functional excipient for oral sustained-release formulations. The excipient is polyvinyl alcohol (PVA)-based and fully synthetic-according to EMD Millipore, this feature ensures batch-to-batch and performance consistency and facilitates quality by design (QbD) and validation processes.

PTSM: Pharmaceutical Technology Sourcing and Management
Hovione is investing in specialized formulation capabilities, beginning with the acquisition of a formulation facility adjacent to the current process chemistry and particle engineering facility in Loures, Portugal, the company announced on Oct. 19, 2015. This acquisition is a strategic investment to further boost development and manufacturing capabilities for both inhalation and oral dosage forms.

PTSM: Pharmaceutical Technology Sourcing and Management
At CPhI Worldwide 2015, The Dow Chemical Company announced the global commercial availability of AFFINISOL HPMC HME, a new generation of cellulosic polymer for drug solubilization. The polymer is designed for use by pharmaceutical companies looking to enhance the solubilization and inhibit the recrystallization of APIs in hot-melt extrusion (HME) formulations.

PTSM: Pharmaceutical Technology Sourcing and Management
CDMO Aesica Pharmaceuticals introduced a new service approach in which the company will taks on the management of other CDMOs, while serving as the single, central point of customer contact, the company announced in an Oct. 14, 2015 press release.

PTSM: Pharmaceutical Technology Sourcing and Management
Ajinomoto Althea, a biopharmaceutical CDMO, is expanding its existing biological drug product manufacturing operations to include highly active materials, such as antibody-drug conjugates (ADCs), the company announced on Oct. 14, 2015. The new facility is located in close proximity to the company’s existing operations in San Diego, CA.

PTSM: Pharmaceutical Technology Sourcing and Management
In the past, contract manufacturing organizations (CMOs) and contract development manufacturing organizations (CDMOs) have been focused most on achieving scale, reaching new clients in new geographic regions, and adding services that were previously unavailable, according to Gil Y. Roth, president of the Pharma and Biopharma Outsourcing Association. In a new report from CPhI, Roth explicates how consolidation has changed how CMOs position their services to clients and how CMOs add value to their businesses.

PTSM: Pharmaceutical Technology Sourcing and Management
Grace and Formac Pharmaceuticals have announced SilSol 6035 Mesoporous Silica Gel, the first silica in the Grace silica-based drug delivery platform. The silica gel is the result of a multi-year collaboration between the companies.The platform was engineered to offer a new drug delivery option for APIs with poor aqueous solubility (BCS class 2 compounds), Grace reports and combines the company’s expertise in mesoporous silica gel and novel application methods to accelerate the screening and development of stable, amorphous-based systems.