
Getting the Truth Out of Dissolution Testing

Getting the Truth Out of Dissolution Testing

AAPS President offers hope and solutions for the industry's challenging future.

Is the square root of (N) + 1 a statistically valid scheme?
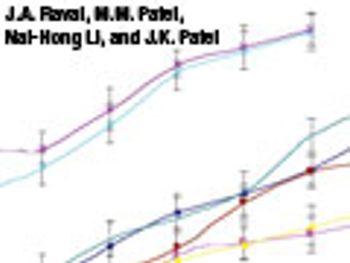
The authors investigated the effects of formulation and processing parameters on floating matrix-controlled drug-delivery systems.

Pharmaceutical companies in India have had a hold on the biotechnology sector for many years, and they're not about to let the follow-on biologics market pass them by.

The countries of Central and Eastern Europe and the Commonwealth of Independent States are closing in on global pharmaceutical competition.

Strict enforcement, new rules, and organizational changes signal an activist tone.

The authors relay the outcome of a two-day workshop that brought together regulators and generic-drug industry representatives.

Industry, equipment vendors, and regulators are busy refining the precision and reliability of dissolution testing.
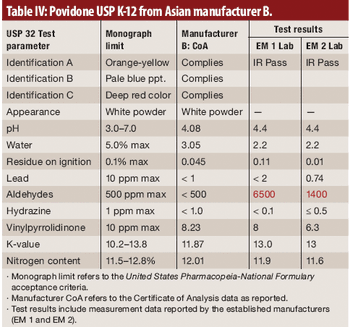
This article demonstrates that test results support the position of FDA on the importance of an appropriate supplier-qualification program.

Using handheld Raman spectroscopy, methods were developed and evaluated for 198 substances widely used as raw materials.

BIO supports recent Congressional action toward a 12-year data exclusivity period for innovators.

The nation's healtcare system needs an overhaul, but it has to be done right.

Like life, the workplace also can have many surprises.

Editors' Picks of Pharmaceutical Science & Technology Innovations

New nanotechnology-based delivery systems offer promise in drug delivery, particularly for anticancer therapeutics.

FDA has been encouraging drug sponsors to use a systematic approach such as quality-by-design principles for pharmaceutical development.

Brief pharmaceutical news items for October 2009.

Representatives of one pilot program participant, Wyeth, outline the experiences and lessons learned for implementing a science- and risk-based approach to drug-development and manufacturing.

A new analysis highlights growth drivers and challenges for clinical supply-chain services.

The author discusses control strategies via near infrared instrumentation for continuous mixing, granulation, drying, and extrusion with a more focused detail on mixing.

Officials from the US Food and Drug Administration discuss best practices for applying quality-by-design concepts. This article contains bonus online-exclusive material.

Contract research organizations such as Covance are heading further east through Europe.
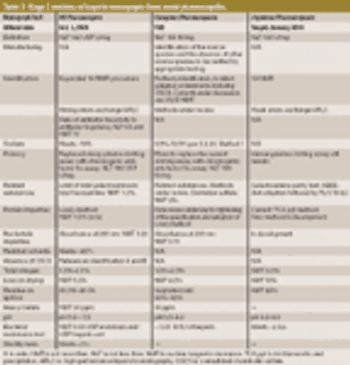
USP workshop participants support new methods to safeguard heparin products but desire international harmonization. This article contains bonus online-exclusive material.

A review of NIPTE's core projects and its plans for training-and retraining-the pharmaceutical industry.

This article introduces the "Q.U.E.S.T." approach for vendor qualification, a practical and compliant methodology for pharmaceutical and biopharmaceutical companies to qualify vendors and hence make well-informed purchasing-related decisions.

As the pharmaceutical industry moves further into Central and Eastern Europe and the Commonwealth of Independent States, several standard-setting and regulatory bodies are also increasing collaboration in the region, particularly in Russia.

The author describes the framework needed to implement QbD and achieve the deeper process understanding that is fundamental to QbD.

This article is part of a Special Report on the Emerging Markets of The East, October 2009

Pharmaceutical companies get ideas for sustainable packaging from other industries.