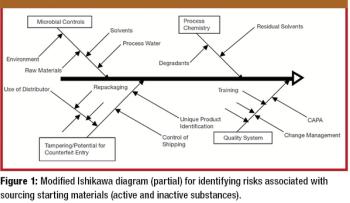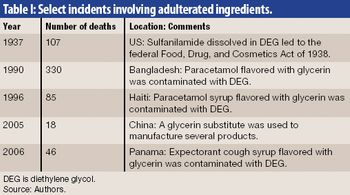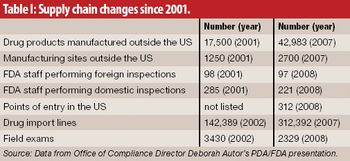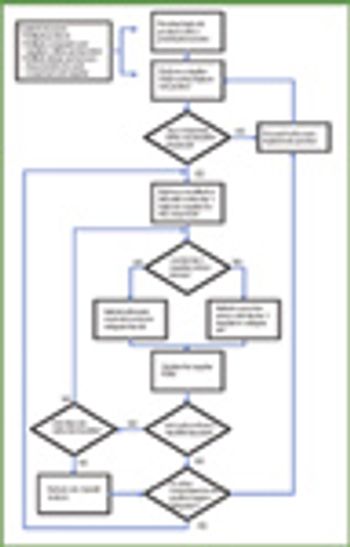
The growth and globalization of the pharmaceutical supply chain make risk assessment more important than ever for pharmaceutical manufacturers. The authors describe a program to identify, prioritize, mitigate, and communicate risks in manufacturer–supplier relationships.