
Electronic pharmaceutical tablet counters meet demands for accuracy, flexibility, speed, compact size, easy cleanability, and quick changeover.

Electronic pharmaceutical tablet counters meet demands for accuracy, flexibility, speed, compact size, easy cleanability, and quick changeover.
Biologics exhibit greater variability in stability testing than do small-molecule drugs, and maintaining a stable test environment is crucial.

Manufacturers and FDA look for innovative strategies to meet accelerated timeframes.
FDA, Congress, and early adopters look to speed up the use of continuous API manufacturing.

The European Union has a challenging task ahead as it strives to harmonize regulations on advanced therapy medicinal products.

Modern methods and modeling offer a better way to understand solubility issues and solve today’s complex formulation challenges.
Prodrugs and drug-delivery systems controlled by time, pH, and osmosis, are being used to prevent drug degradation in the stomach and large intestine and ensure drug release in the colon.

Siegfried Schmitt, principal consultant, PAREXEL, discusses how to handle internal audit reports during inspections.

Heightened regulatory scrutiny of data integrity highlights the need for comprehensive procedural reviews and strategies for managing mission-critical information.

Pharmaceutical Technology spoke with Gerard Creaner, president of GetReSkilled, about the the Behavioral Positioning System.
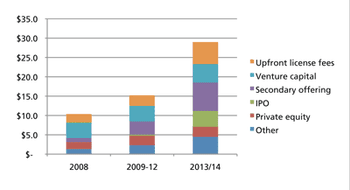
While all market signs are pointing up, memories of past setbacks may discourage from expanding capacity.

The bio/pharma industry enjoys success, but it cannot ignore patient access to medications.
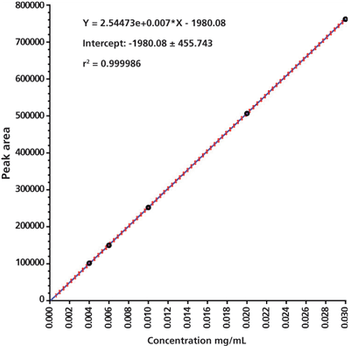
Statistical procedures give statistical answers not analytical judgement.

PTI Inspection System’s VeriPac 310 leak detection and package integrity testing system reduces waste and provides the user with a clear evaluation of package integrity.

Ross’ Double Planetary Mixer lined with Teflon is designed for high-purity applications and features resistance to chemicals and corrosive materials when stainless steel is not compatible.

Anton Paar’s MCP polarimeters are now designed with a new LED light source and optional integrated air pump to clean and dry sample cells.

Thermo Scientific’s multimode microplate reader, the Varioskan LUX, is designed with intuitive software to allow for automatic and versatile functionality when performing microplate assays.

With the French pricing and reimbursement policies becoming increasingly stringent, pharmaceutical manufacturers must adapt their drug development and commercial strategies if they want to secure premium pricing for their new products.
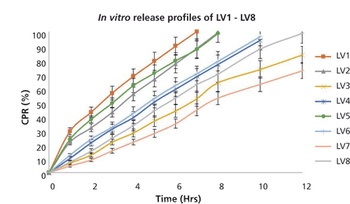
Delivery systems that allow drugs to be administered as liquids, but to form gel within the eye, promise to improve efficacy and patient compliance.

Click the title above to open the Pharmaceutical Technology July 2015 issue in an interactive PDF format.

An ISPE guidance document, four years in the making, brings risk-based thinking, statistics, and Lean Six Sigma to cleaning validation.