
Bills to regulate drug compounding and establish a national track and trace system face political and policy differences.

Bills to regulate drug compounding and establish a national track and trace system face political and policy differences.

Roberto Darienzo, chief operating officer of Halo Pharmaceutical, describes strategic plans and industry trends.
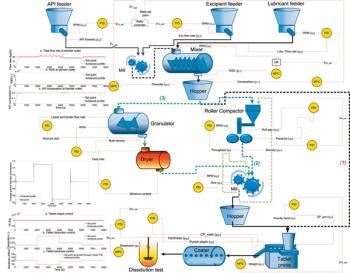
Applying model-predictive methods and a continuous process-control framework to a continuous tablet-manufacturing process.
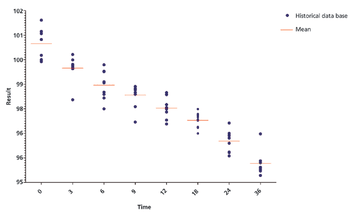
The authors discuss three methods for identification of out-of-trend (OOT) results and further compare the z-score method and the tolerance interval in OOT analysis for stability studies.

A science- and risk-based approach to verify and demonstrate that a process operating within predefined specified parameters consistently produces material that meets all its critical quality attributes.

A unique demographic and payer mix make ASEAN an increasingly attractive region.

Companies can use metrics as a tool to help drive positive change and quality process improvements.

Editor's picks of pharmaceutical science and technology innovations in manufacturing and equipment.
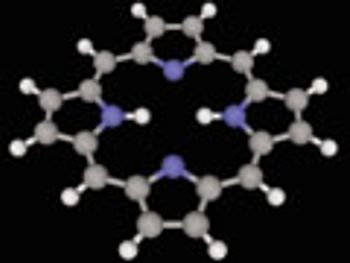
Researchers at the Scripps Research Institute advance heteorcylic chemistry trhough new reagents and reaction-tracking techniques.

A recent study of computer-created and natural proteins suggests the number of sites where small-molecule drugs can bind to proteins is limited, thereby narrowing how to mitigate side effects through drug design.

Ranbaxy's $500 million settlement for producing adulterated drugs and fradulent data provides a cautionary tale for patients, FDA, and drug manufacturers.

Models and modeling software gain a foothold in solid-dosage manufacturing process design.

Crushing, fracturing, and bending tests quantify hardness.

Siegfried Schmitt discusses the European Commission's (EC) guidelines on Good Distribution Practice of Medicinal Products for Human Use

A multifaceted approach is needed to resolve the myriad of challenges in developing oral formulations of poorly soluble drugs.

The Taiwanese government engages in regulatory science in a move to boost its pharmaceutical sector.
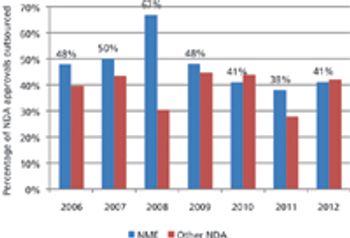
Approvals of new drugs are on an upward swing, but only a few CMOs are benefiting.

Microwave spectroscopy overcomes some of the limitations of circular dichroism and vibrational circular dichroism in analyzing enantiomers.

An overview of the latest regulatory developments for malaria drugs, biosimilars, and global standards.
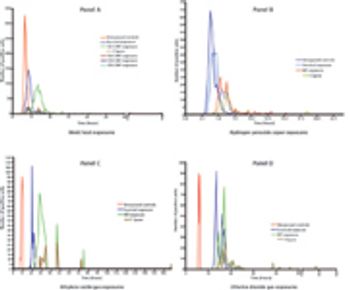
The authors report on the range and distribution of grow-out times for biological indicators exposed to sublethal sterilization processes.

Kurt Nielsen, PhD, chief technology officer and senior vice-president of R&D at Catalent Pharma Solutions, discusses strategies for solubility and bioavailability enhancement of poorly water-soluble drugs.

Click the title above to open the Pharmaceutical Technology June 2013 issue in an interactive PDF format.