
Bio/pharmaceutical manufacturers in the UK face challenges in proving that their drugs are worth the price.

Bio/pharmaceutical manufacturers in the UK face challenges in proving that their drugs are worth the price.

Risks associated with single-use technologies, such as product loss and sterility assurance, are magnified in the filling operation because of its closeness to the product in its final form. A thorough evaluation of the assembly design process, manufacture, and use can assist in identifying and mitigating these risks.
New materials and software modeling streamline the blister-package design process.
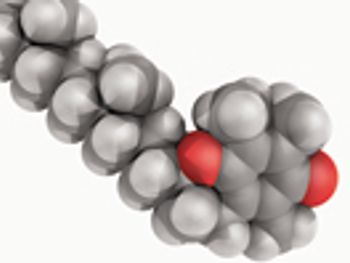
Tocophersolan or TPGS was developed 60 years ago as a water-soluble form of vitamin E. The author gives an overview of TPGS, including its interesting properties, examples found in the literature, and a brief summary of the regulatory status and marketed formulations.

Fred Miesowicz, chief operating officer of Argos Therapeutics, discusses the advantages and challenges of developing and manufacturing personalized immunotherapies.


The European Commission’s new structure has sparked controversy about its allocation of responsibilities and the impact on the development and approval of new medicines.
Understanding and preventing protein aggregation is crucial to ensuring product quality and patient safety.


In late 2014, standards organizations continued to work towards harmonization and securing drug safety.

Manufacturers are under pressure to develop pipelines, promote quality, and justify pricing.



Changing dynamics of the pharmaceutical industry are driving demand.
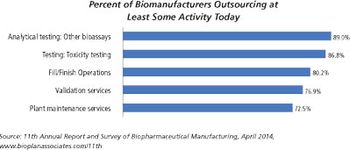
There are significant differences between small molecules and biologics fill/finish capacity.

Siegfried Schmitt, Principal Consultant, PAREXEL International, discusses how to handle staff challenges to regulation requirements

The choice of dilution schemes can minimize solution preparation errors.

New designations lead to faster drug approvals, but there is more work to be done.

Click the title above to open the Pharmaceutical Technology January 2015 issue in an interactive PDF format.