
Equipment and Processing Report
INTERPHEX 2017 exhibitors focus on shelf life, traceability, and quality control.

Equipment and Processing Report
INTERPHEX 2017 exhibitors focus on shelf life, traceability, and quality control.
Equipment and Processing Report
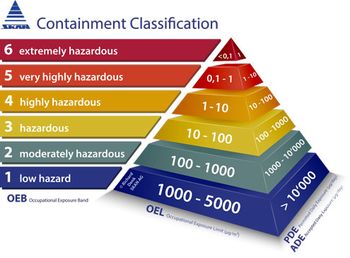
The new ISPE Containment Manual is a summary of the process involved in the manufacture of highly active or highly hazardous pharmaceutical substances.

Equipment and Processing Report
A Keynote Series includes presentations by experts in serialization and traceability, continuous solid-dosage manufacturing, cleaning validation, and post-approval changes.