
Several steps need be taken to achieve lights-out, fully automated operations.
Richard Denk is Head Sales Containment at SKAN AG, www.skan.ch, Richard.Denk@skan.ch.

Several steps need be taken to achieve lights-out, fully automated operations.

Decontamination, automation, and containment are important considerations for aseptic manufacturing in isolators.

An optimal engineering design is crucial for aseptic operation and cleaning.
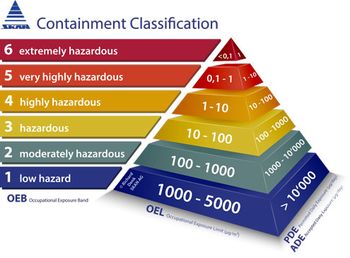
The new ISPE Containment Manual is a summary of the process involved in the manufacture of highly active or highly hazardous pharmaceutical substances.

The new ISPE Containment Manual is a summary of the process involved in the manufacture of highly active or highly hazardous pharmaceutical substances.

Manufacturing of antibody drug conjugates requires high-containment solutions, such as high-performance aseptic isolators.

Published: November 3rd 2020 | Updated:

Published: May 15th 2022 | Updated:

Published: April 3rd 2021 | Updated:

Published: February 18th 2016 | Updated:
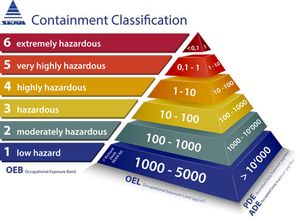
Published: March 2nd 2017 | Updated:

Published: March 15th 2017 | Updated: