
Determining E&L risk from single-use components can be used to build the level of extractable profiling and PERLs.
Tathagata Ray is Area Manager, Process Development at Millipore India Pvt. Ltd (India).

Determining E&L risk from single-use components can be used to build the level of extractable profiling and PERLs.

The evolution of therapeutic modalities drives the adoption of single-use technologies.
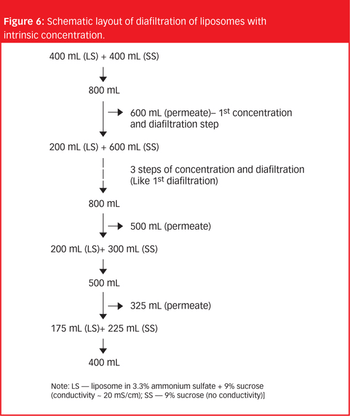
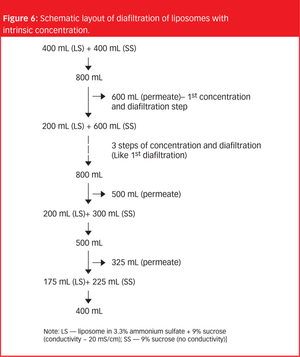
The importance of liposomes as an effective drug delivery system is well accepted in the pharmaceutical industry, but their handling remains a challenge.

Published: March 16th 2024 | Updated:
Published: June 1st 2009 | Updated: