
This morning I received a press invitation from Boehringer Ingelheim for the beta launch of its Facebook game, Syrum. The game is the first of its kind to be developed by a pharmaceutical company.
Stephanie Sutton was an assistant editor at Pharmaceutical Technology Europe.

This morning I received a press invitation from Boehringer Ingelheim for the beta launch of its Facebook game, Syrum. The game is the first of its kind to be developed by a pharmaceutical company.

The European Medicines Agency has recommended that the anticancer medicine DepoCyte be recalled from EU countries following the discovery of manufacturing deficiencies at Pacira Pharmaceuticals' San Diego site.

GlaxoSmithKline (GSK) is selling the majority of its 'classic brands' in Australia to Aspen Global for approximately £172 million ($271.1 million) in cash.

AstraZeneca has paid approximately $3.2 billion to extend its diabetes alliance with Bristol-Myers Squibb (BMS), following BMS's recently completed acquisition of Amylin Pharmaceuticals.

The UK is to invest £8 million ($12.5 million) in a new center that will be dedicated to stem-cell biology and medicine, with the aim of developing new therapeutic approaches to illnesses that currently have no effective treatments.

My daughter has been learning how to ride a bike for the past year. She’s actually become quite adept at it, and for someone who loves to cycle, I am certainly proud. But there were certain obstacles to overcome to get to this point. We started simply with the tricycle – learning to pedal.

Emerging markets continue to attract the attention of pharmaceutical companies thanks to their high growth and large patient populations.

Novartis has entered into a global collaboration with the University of Pennsylvania in the US to research, develop and commercialise targeted cancer therapies.

A report from the European Commission shows that fake pharmaceuticals were the top articles detained by European Union customs in 2011.

The European Commission has issued a statement of objections against Lundbeck and several other pharmaceutical companies with the preliminary view that Lundbeck formed agreements with generic-drug companies to prevent the market entry of cheaper versions of its antidepressant, citalopram.

The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) recently launched a 12-week public consultation for a national scheme that will look at the possibility of providing seriously ill patients with access to unlicensed medicines still in Phase III, or possibly even Phase II, clinical trials

The UK's Medicines and Healthcare products Regulatory Agency has launched a public consultation regarding a scheme that could provide patients with access to unlicensed medicines in Phase II or III clinical trials.

The European Commission is looking to provide Sweden with EUR4.3 million to help 700 workers affected by AstraZeneca job cuts to find new work.

Experts share insights into analytical tools and techniques.

GlaxoSmithKline has announced that it will acquire Human Genome Sciences (HGS) for $14.25 per share in cash, or approximately $3.6 billion on an equity basis.

There are many ways in which pharmaceutical companies can make a contribution to society beyond developing and manufacturing medicines.

Experts share how to choose analytical tools and techniques when scaling up a lyophilization process.
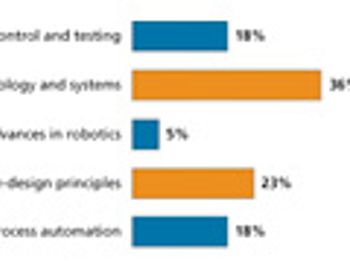
Readers point to quality by design as having a significant influence on manufacturing and drug development during the past decade.

Last week, I looked at a consultation in the UK concerning access to unlicensed medicines.

The European Medicines Agency has published its first set of finalized guidelines concerning good pharmacovigilance practices.

The European Medicines Agency has launched an investigation into Roche after an inspection found that thousands of potential safety reports, including 15161 deaths, connected to Roche medicines had not been evaluated to determine whether they should be reported to regulators as adverse drug reactions.

A coordinated European programme was recently carried out involving routine inspections of pharmaceutical companies’ safety reporting systems.

Merck Serono has revealed its final "final efficiency program" for operations in Switzerland, which includes confirmation of the closure of sites in Geneva and Coinsins and their associated job cuts.

The European Federation of Pharmaceutical Industries and Associations has called for the German government to take "urgent action" regarding its pricing system, which is perceived as hindering the market entry of new, innovative medicines in the country.

Germany used to be a golden market for the pharma industry in Europe because the country allowed global pharmaceutical companies to set their own prices for new medicines. However, all good things must come to an end…

Dr. Reddy's Laboratories and Merck Serono, a division of Merck KGaA, have partnered to codevelop a portfolio of biosimilar compounds in oncology.

UK chooses to use off-label drug indication to cut healthcare costs. Will others follow suit?

The European Federation of Pharmaceutical Industries and Associations (EFPIA) has welcomed the launch of a EUR 223.7-million ($276.5 million) program to tackle antimicrobial resistance and speed up the development of new antibiotics.

A recent report from the UK's All Party Pharmacy Group has attributed shortages of prescription medicines in the UK to parallel trade and is calling for urgent action.

A bill has recently been in discussion in the US that contains measures to strengthen the ways in which drug shortages are dealt with. The US FDA is also taking action by asking companies to alert them as to possible drug shortages so that it can prepare back ups.