Articles by Stephan O. Krause
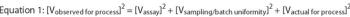
The first part of this article discussed general strategies for validation extensions to other test method components, laboratories and even different test methods.1 This second part provides practical tips on how to maintain test method suitability long after the formal completion of analytical method validation (AMV) studies.

Firms can use practical tools and criteria to implement advanced analytical test methods for biopharmaceuticals. They an also extend and maintain the validation status for a licensed test method from an understanding of the relationship of historical process data, specifications and test method capability.