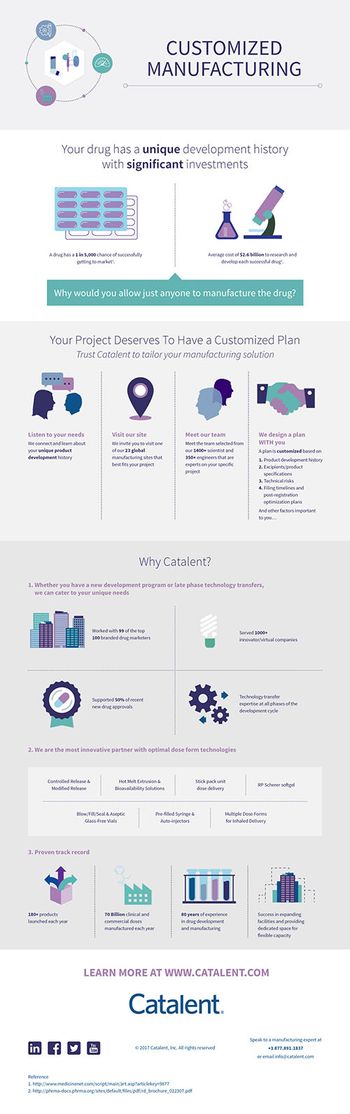
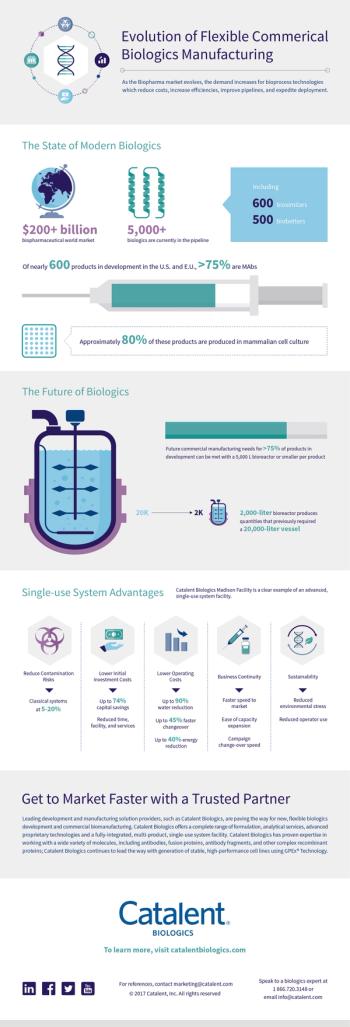
Sponsor's Content
Advertisement
Articles by Sponsor's Content
Advertisement
Latest Updated Articles
 Best Practices for Product Transfer
Best Practices for Product TransferPublished: May 18th 2016 | Updated:
 Bio Pharma Reforms in China
Bio Pharma Reforms in ChinaPublished: May 25th 2016 | Updated:
 Biopharma in China: New Initiatives, New Opportunities
Biopharma in China: New Initiatives, New OpportunitiesPublished: June 17th 2016 | Updated:
 A Confirmatory Method for PCDDs and PCDFs
A Confirmatory Method for PCDDs and PCDFsPublished: June 20th 2016 | Updated:
 Pesticide Screening of Food Samples Using the ionKey/MS System
Pesticide Screening of Food Samples Using the ionKey/MS SystemPublished: June 20th 2016 | Updated:
 How to Scale-up a Hot Melt Extrusion
How to Scale-up a Hot Melt ExtrusionPublished: June 20th 2016 | Updated:
Advertisement
Advertisement
Trending on Pharmaceutical Technology
1
PharmTech Weekly Roundup - February 6, 2026
2
Drug Digest: Outsourcing Partnerships Fuel Global Biopharma Discovery and Scale-Up
3
Women in STEM: Cultivating Scientific Confidence
4
Rare Disease Treatments: Navigating the Economics of Global Innovation
5