
Novartis Sells US Enablex Rights; Ricerca Names Chemistry Director; And More.

Novartis Sells US Enablex Rights; Ricerca Names Chemistry Director; And More.

USP to Make Pharmacopeial Forum Free Online, And More.

DSM and PolyTherics in Development Deal; Kite Pharma Appoints President and CEO, And More.

The US Food and Drug Administration recently issued a Warning Letter to Bristol-Myers Squibb (BMS, New York) for violations of current good manufacturing practice at the company's Manati, Puerto Rico, manufacturing facility.

Genzyme Sells Genetics Business; Bausch and Lomb Names Vice-President; and More.

Last week, Lonza (Basel) agreed to support the ongoing development of GlaxoSmithKline?s (GSK, London) biopharmaceutical pipeline by supplying manufacturing capacity for five early-stage monoclonal antibodies.

ISPE to Hold Risk-MaPP 2010 Conference

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the September 2010 edition from Alfa Laval and Gems Sensors and Controls.
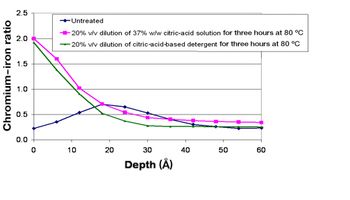
The author describes the types and sources of rouge and explains ways to prevent and mitigate this problem.

US Pharmacopeia apparatuses for testing the dissolution of transdermal drugs produce good, reproducible results. Yet some scientists believe that further modifications could improve the instruments? suitability for this application.

Pfizer to Acquire FoldRx; Protalix Appoints COO; And More.

Roche (Basel) launched a groupwide operational-excellence initiative intended to modify the company's cost structures and accelerate its productivity improvements.

ICH to Hold Training Workshops for Quality Guidelines

FDA Provides Information On Advisory Committees, Announces Public Meetings, And More.

Genzyme (Cambridge, MA) confirmed that it had received sanofi-aventis's (Paris) proposal to acquire all of its outstanding shares for $69 each.

A roundup of developments on corporate social responsibility from the bio/pharmaceutical industry, its suppliers, and other public and private organizations.

Week of Aug. 23, 2010: Company and People Notes: Quark and Novartis Form Agreement; Hospira CEO Retires; And More.

Regulatory Roundup: PDUFA Fees Published, And More.

Two new reports describe the vaccine market's recent growth and predict future market expansions.

Eli Lilly Ends Semagacestat Program; Selecta Appoints CEO; And More.

Commissioner Hamburg Says China is Improving Export Regulation

PharmTech's monthly newsletter, Equipment and Processing Report, reviews the Editor's Picks for the August 2010 edition from Lechler and Thermo Fisher Scientific.

Making highly potent active pharmaceutical ingredients (HPAPIs) can be costly because the process often requires equipment specialized to achieve containment and extra attention to safety concerns. Pharmaceutical professionals may wonder whether disposable components, which have reduced the cost of some operations, might be appropriate in the manufacture of HPAPIs.

This week, the US Food and Drug Administration published on its website a summary of good manufacturing practices for the holding and distribution of drug products.

Penwest and Endo to Merge; Watson Makes Senior Appointments; And More.

In a draft guidance published this week, the US Food and Drug Administration recommended that makers of transdermal and transmucosal drug-delivery systems use "an appropriate scientific approach" during product design, development, and manufacturing to minimize the amount of residual drug substance present at the end of the products' labeled use periods.

FDA Approves Flu Vaccines; Caraco Names COO; And More.

A roundup of developments on corporate social responsibility from the bio/pharmaceutical industry, its suppliers, and other public and private organizations.

Merck and Sinopharm Sign Agreement; Agilent Appoints CFO; And More.

In 2009, the European Commission (EC) Customs Union seized 11,462,533 medicines and medical products for suspected violations of intellectual property (IP), according to the Commission's annual report on EU Customs Enforcement of Intellectual Property Rights, which was published last week.