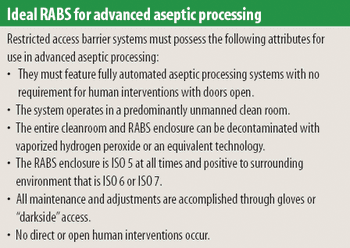
Any aseptic processing technology that allows intervention by gowned personnel during operation cannot be considered an advanced technology. Although a standardized definition of restricted access barrier systems has been developed, these systems fall well short of being classfied as advanced technologies.


