
Alternative drug delivery approaches are promising, but due to their complexity, they need to be sufficiently justified.
Felicity Thomas is the former Associate Editorial Director of Pharmaceutical Technology, Pharmaceutical Technology Europe, BioPharm International.

Alternative drug delivery approaches are promising, but due to their complexity, they need to be sufficiently justified.

Manufacturing capacity expansion is high on the agenda for many bio/pharma companies and service providers in Europe.

Reformulation strategies are useful tools for more than just stretching out the potential return on investment for a product.
There are expectations that changes to pharma regulations in the EU, forming part of the regions broader strategy for the industry, will be positive and should offer flexibility for industry to advance through innovation.
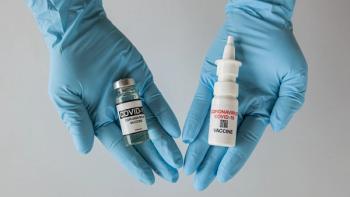
The intranasal route of administration is showing clinical promise, particularly for COVID-19, but there are multiple hurdles to overcome to ensure successful formulation.

Dosage form priorities are shifting to focus on user-friendliness, leading to greater engagement with outsourcing partners earlier in development timelines.

Collaborative partnerships can foster success in formulation development projects.

Pharma and biotech supply chain companies are working together to help facilitate the transition of the pharma industry to net zero emissions compliance.

Excessive drug pricing is being scrutinized across Europe, as hefty fines are set to send out a clear message of intolerance to anti-competition conduct.

Recent research has highlighted the underlying mechanisms of amorphous solid dispersions and theory behind the formation of drug-rich phases.

There are some key questions that should be asked by both the sponsor company and the outsourcing partner before undertaking a method development project.

Bi-layer tablets are an under-utilized option that can be employed to help reduce treatment burden, but their formulation is more complex than for conventional monolayer products.

The draft revisions of Annex 1 are driven by a quality risk management approach and will provide more clarity and detail for manufacturers.

Five therapeutic candidates for the treatment of COVID-19 are identified as promising by the European Commission.

Pharmaceutical Technology discussed the potential increased risk of counterfeit medicines in the UK post-Brexit and how blockchain could be a useful tool to tackle the issue.

Thought and foresight into method development stages can ensure costly errors and delays are avoided later on.
Particle engineering is a vital tool in overcoming many formulation challenges, and technological advances are enabling developers to achieve the full potential of pipeline molecules.

Outsourcing method development offers multiple benefits to companies, including access to experience and expertise, streamlined costs, and development time efficiencies.

GSK’s position is raising concerns as it continues working towards its separation of businesses.
Certain therapeutics, such as ophthalmics, must be provided as a sterile dosage form but can pose fill/finish challenges due to the small batch sizes required and the fact that the products used are difficult to fill and of high value.

Despite its importance in drug development, dissolution testing still has some limitations, but advances in automation and real-time monitoring are producing promising results.

Complex formulations, personalized medicines, COVID-19 therapies, and sustainability goals are driving innovations in drug packaging.

Vaccine development is inherently challenging; however, in light of the COVID-19 pandemic, innovations have been prioritized, leading to accelerated development processes.

Accelerated formulation strategies are a useful tool to reduce development timelines and cost, but key priorities must be considered early on to ensure success.

After Brexit there is an increased risk of the UK being exposed to counterfeit medicines, but regulations implementing blockchain as infrastructure technology could be the answer.

Despite its importance in drug development, dissolution testing still has some limitations, but advances in automation and real-time monitoring are producing promising results.

COVID-19 has placed bio/pharma in the spotlight of global media, but away from vaccines there are other prospective pipeline treatments that will be worth watching.

Dosage-level authentication provides an added digital layer of security for pharmaceutical companies to ensure their products are not exposed to falsification.

The Testa Center in Sweden provides access to equipment and expertise to help bridge the gap between biopharmaceutical discovery and industrialization.
Various strategies to improve bioavailability are being continuously evaluated, affording greater commercial prospects for the future.