
President-elect Barack Obama officially nominated former Senator Tom Daschle as Secretary of Health and Human Services and named him the Director of a new White House Office on Health Care Reform late last week.
Angie Drakulich was editorial director of Pharmaceutical Technology.

President-elect Barack Obama officially nominated former Senator Tom Daschle as Secretary of Health and Human Services and named him the Director of a new White House Office on Health Care Reform late last week.

The Federal Trade Commission (FTC) is seeking public comment on proposed revisions to its Guides Concerning the Use of Endorsements and Testimonials in Advertising.

The US Food and Drug Administration issued a draft guidance, Contents of a Complete Submission for the Evaluation of Proprietary Names, on Nov. 24, 2008.

The US Food and Drug issued a draft guidance on Tuesday, Nov. 18, titled Process Validation: General Principles and Practices for comment. The guidance is meant to serve as a revision to the 1987 Guideline on General Principles of Process Validation.

The US Food and Drug Administration issued a direct final rule last week to adjust for inflation the maximum civil money penalty amounts for various civil money penalty authorities under the agency's jurisdiction.

Rep. John Dingell (D-MI) plans to introduce the Food and Drug Globalization Act discussion draft as a bill early next year.

The Steering Committee and Expert Working Groups of the International Conference on Harmonization are meeting this week in Brussels to discuss current harmonization efforts.

The US Food and Drug Administration has issued a final rule requiring the addition of a statement and toll-free number to the labeling of certain human drug products.


The US Government Accountability Office (GAO) released a report last week that examines and provides recommendations for the US Food and Drug Administration's foreign inspection process, including the agency's data management, inspection frequency, and oversight of problems identified during inspections.

Merck plans to cut approximately 7200 positions as part of its 2008 restructuring plan, according to an Oct. 22 release focused on the company's third-quarter financial results.

The US Department of Health and Human Services (HHS) will send the first Food and Drug Administration staff to China, India, Europe, and Latin America before the end of 2008.

FDA has completed its labeling updates to fluoroquinolone antimicrobial drugs.

During the past several months, Pharmaceutical Technology has been covering the US Food and Drug Administration's rulemaking on over-the-counter (OTC) cough and cold medications for children.

The lack of appropriate financial resources for the US Food and Drug Administration's growing global agenda has been a pertinent topic of late?and now appropriate staffing has become a large concern.

The US Food and Drug Administration?s Center for Drug Evaluation and Research (CDER) held a public meeting on Oct. 2 to discuss over-the-counter (OTC) cough and cold medications for pediatric use.

The electronic pedigree mandate for prescription drugs has been delayed again. California Gov. Arnold Schwarzenegger signed legislation that delays ePedigree implementation until 2015.

Advancements add yet another challenge for industry's already overextended regulatory body.

A public meeting is being held today to discuss over-the-counter cough and cold medications for pediatric use.
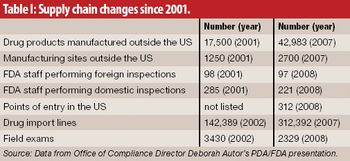
Pharmaceutical Technology has summarized recent statements by FDA officials on supply chain issues to provde the agency's most up-to-date views and expectations.

In a Sept. 17 letter to FDA Commissioner Andrew C. von Eschenbach, Rep. Henry Waxman (D-CA) questions the agency's priorities, specifically poking at FDA's political appointees and whether they are promoting industry at the expense of the public's health.

In November, representatives to the International Conference on Harmonization will meet in Brussels, Belgium, to discuss several international cooperation initiatives, including ICH Q10: Pharmaceutical Quality System and ICH Q8R: Pharmaceutical Development.

Earlier this month, the US Food and Drug Administration announced that it will be posting quarterly reports on its website regarding potential drug safety issues.

The US Food and Drug Administration will hold a public hearing Oct. 2, 2008, to obtain input regarding over-the-counter (OTC) cough and cold drugs marketed for pediatric use.

The deadline for nominations for the Innovations in Pharma Science Awards is here. Check out http://pharmtech.com/Innovations to nominate your company's work in one of five areas by next week

The US Food and Drug Administration may soon ask doctors to undergo special training to be able to prescribe powerful narcotics, Dr. Bob Rappaport told The New York Times last week. Rappaport, director of FDA's Anesthesia, Analgesia and Rheumatology Products division, said the agency is considering recommending additional education for doctors in early 2009.

The US Food and Drug Administration issued a new draft guidance for public comment on sterile manufacturing.

Next week, the US Food and Drug Administration's communications with pharmaceutical companies will change, and the agency will begin issuing complete response letters in place of approvable letters.

The US Food and Drug Administration released its user fee rates for fiscal year 2009 last Friday.