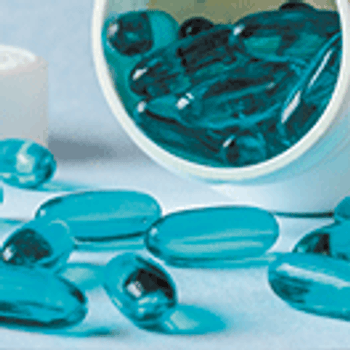
Lipid-based formulations offer a means of addressing the physicochemical and biological challenges of poorly soluble APIs.
Adeline Siew is editor for Pharmaceutical Technology Europe. She is also science editor for Pharmaceutical Technology.

Lipid-based formulations offer a means of addressing the physicochemical and biological challenges of poorly soluble APIs.

Senomyx uses an approach, known as taste-blocking, to address the challenges of bitter APIs. Kenneth J. Simone, vice-president of Pharmaceutical Business Development, Senomyx, speaks with Pharmaceutical Technology about this technology.
The level of tastemasking required will depend on the API properties and the dosage form design.
Immobilizing the antibodies on a solid-phase support, such as a resin, and carrying out the conjugation of the payload-linker while the antibodies are bound to that support will prevent aggregation at its source.

Formulation characteristics must be optimized, following device selection, to achieve the desired performance for the capsule loaded in the dry powder inhaler.
Materials in contact with a drug must be fully characterized to ensure they do not negatively affect the safety and efficacy of the product.

Jean-Yves Balfin, product manager at Korsch AG, speaks to Pharmaceutical Technology about the ins and outs of multilayer tableting.

New approaches enable more patient-centric drug design that offers improved outcomes.

The CUREfilm technology delivers drugs through a dissolvable strip that can be applied in the oral cavity or onto the skin.

Choice of carrier can have a significant impact on the capsule filling process as well as the performance of the DPI formulation.

Determining the right process conditions for a freeze-drying cycle requires an understanding of the effect of each step on the drug product.
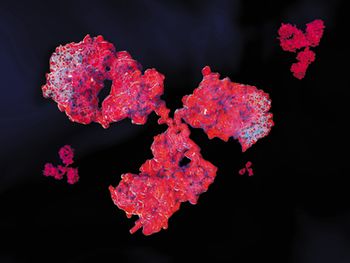
The high viscosity of highly concentrated monoclonal antibody formulations not only presents processing and storage difficulties, but also formulation and delivery challenges.

New technologies, such as NIR and Raman, enable online measurements of blending and content uniformity in the production of solid dosage forms.

Experts share insights on the various methods used for purity and impurity analysis of therapeutic proteins.

Mark Egerton, PhD, chief executive officer of Quotient Sciences, shares insights on a new approach to accelerate drug development, which integrates formulation development, real-time adaptive GMP manufacturing, and clinical research within a single platform.

Graham Reynolds, vice-president and general manager, Global Biologics at West Pharmaceutical Services, Inc., shares insights on key considerations when developing a biologic drug product in a prefilled syringe.

Abuse-deterrent opioid formulations generally fall into two categories: the first is based on a physiochemical abuse-deterrent approach and the second combines the opioid with an antagonist.

The development of new packaging solutions that are fit for drugs of the future requires close collaboration between the pharmaceutical manufacturer and its packaging suppliers and machine vendors.

Measurements by a drone-based online pressure monitoring system help identify weak points in the filling line, enabling the process to be optimized.

Glass and plastic are well established primary packaging materials for the pharmaceutical industry but they both have their advantages and disadvantages.

The method developed by Cambrex uses standard plant equipment and requires only two synthetic steps and one recrystallization, minimizing waste and use of solvents.

Orbis Biosciences’ Optimµm Platform delivers microparticulate dosage forms with controlled-release and taste-masked properties in a single manufacturing step.

Softgel capsules are a popular dosage form among patients but they also provide a number of manufacturing benefits over liquid-filled hardgel capsules.

Modified-release lipid-based formulations in softgel capsules can address physiochemical and pharmacokinetic challenges posed by drug compounds.

Pharma trends translate into increased need for not only external partners, but those will capabilities that can help advance today’s drug development and manufacturing challenges.

Ralph Gosden, head of product development at Catalent’s Swindon, United Kingdom site, explains the opportunities of orally disintegrating tablets as a patient-centric dosage form.

Experts from Colorcon share insights on how manufacturers can play a role in minimizing the risk of medication errors by ensuring that the medicines they develop are well differentiated, especially between dosages of the same product.

Pharmaceutical companies are increasingly adopting patient-centric formulation development to ensure that drug products adequately meet the needs of end-users in all target patient populations.

The pharmaceutical industry is under tremendous pressure to make drug development faster and cheaper. Applying the right formulation strategy using structured and rigorous science can help avoid costly failures and re-starts, but it’s important to start from an early stage.

Applying the right formulation strategies early in the drug development process can help avoid costly late-stage failures.

Published: January 8th 2013 | Updated:

Published: March 19th 2013 | Updated:

Published: January 16th 2013 | Updated:

Published: March 20th 2013 | Updated:

Published: February 26th 2013 | Updated:

Published: January 7th 2013 | Updated: