
PTSM: Pharmaceutical Technology Sourcing and Management
New catalysts show promise for pharmaceutical intermediate and API synthesis.

PTSM: Pharmaceutical Technology Sourcing and Management
New catalysts show promise for pharmaceutical intermediate and API synthesis.

PTSM: Pharmaceutical Technology Sourcing and Management
Covert and layered approaches to combat drug counterfeiting and illegal diversion strive to stay ahead of criminal enterprises.
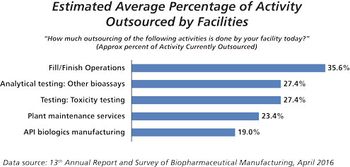
PTSM: Pharmaceutical Technology Sourcing and Management
Automation, single-use systems, smaller footprints, and new drug delivery devices define trends for fill/finish outsourcing.

PTSM: Pharmaceutical Technology Sourcing and Management
Governments stretch limited resources to track counterfeit, diverted, and stolen pharmaceuticals globally. \

PTSM: Pharmaceutical Technology Sourcing and Management
The Czech Republic drug manufacturer was cited for data integrity and quality issues.

PTSM: Pharmaceutical Technology Sourcing and Management
GENCO, a FedEx Company, introduces a multi-tenant warehouse model in Milton, Ontario, and Memphis, Tennessee.

PTSM: Pharmaceutical Technology Sourcing and Management
Catalent invests $34 million to add a 2 x 2000-L single-use bioreactor system and laboratory space in Madison, WI.

PTSM: Pharmaceutical Technology Sourcing and Management
The researchers were awarded the Thomas Alva Edison Patent Award for a solventless method for coating APIs.

PTSM: Pharmaceutical Technology Sourcing and Management
The new guidelines will address bioanalytical method validation and biopharmaceutics classification system-based biowaivers.

PTSM: Pharmaceutical Technology Sourcing and Management
Recipharm announced that it has invested 5 million SEK in a new GLP bioanalysis laboratory in Uppsala.

PTSM: Pharmaceutical Technology Sourcing and Management
CordenPharma announced the completion of a new highly potent process bay for APIs at the company’s Colorado facility.

PTSM: Pharmaceutical Technology Sourcing and Management
Recipharm launched a showcase line to demonstrate the company’s new serialization solutions.

PTSM: Pharmaceutical Technology Sourcing and Management
Marken announced the opening of a new depot and logistics operations center in Argentina.

PTSM: Pharmaceutical Technology Sourcing and Management
A report from the European Union Intellectual Property Office shows that the EU loses approximately €10.2 billion a year due to counterfeit medicines.

PTSM: Pharmaceutical Technology Sourcing and Management
Pharma Venture’s approach in tackling supply-chain, logistic, and distribution challenges in the MENA region was recognized at the CPhI Pharma Awards.

PTSM: Pharmaceutical Technology Sourcing and Management
The Koolit Advanced PCM Gel from Cold Chain Technologies provides cold-temperature protection for drugs and vaccines during transport.

PTSM: Pharmaceutical Technology Sourcing and Management
The company invested in a new cGMP facility located in Berlin, Germany.

PTSM: Pharmaceutical Technology Sourcing and Management
he guidance addresses the good manufacturing practice for managing quality in APIs.

PTSM: Pharmaceutical Technology Sourcing and Management
PaizaBio will add aseptic injectable production capacity in Hangzhou, China.