
PTSM: Pharmaceutical Technology Sourcing and Management
Researchers continue efforts to overcome challenges of effective oral delivery of biologic drugs.

PTSM: Pharmaceutical Technology Sourcing and Management
Researchers continue efforts to overcome challenges of effective oral delivery of biologic drugs.
PTSM: Pharmaceutical Technology Sourcing and Management
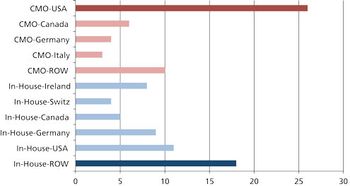
What are the pros, cons, and practicalities of moving pharma manufacturing back to the US? \

PTSM: Pharmaceutical Technology Sourcing and Management
Informatics platforms and automation solutions may support efforts to improve data integrity practices.

PTSM: Pharmaceutical Technology Sourcing and Management
In a pilot program, Johnson & Johnson Supply Chain and AmerisourceBergen demonstrated how data can be transferred between two partners.

PTSM: Pharmaceutical Technology Sourcing and Management
The newly installed Harro Hӧfliger encapsulation unit expands Capsugel’s clinical- and commercial-scale capacity for dry powder inhalation projects.

PTSM: Pharmaceutical Technology Sourcing and Management
RSSL has added new equipment for measuring the surface area of powder particles, which is important for determining the performance of excipients and APIs.

PTSM: Pharmaceutical Technology Sourcing and Management
Permira will acquire LSNE, a CDMO for the pharmaceutical and medical device markets.

PTSM: Pharmaceutical Technology Sourcing and Management
The company has secured an additional facility in Hampton, Middlesex, as part of a project to expand its UK-based operations by 15% in 2017.

PTSM: Pharmaceutical Technology Sourcing and Management
The company has invested in a new pharmaceutical chemistry and microbiology facility in Scotland.

PTSM: Pharmaceutical Technology Sourcing and Management
Caladrius is selling the remaining percentage of the subsidiary in order to focus on cell therapy development.

PTSM: Pharmaceutical Technology Sourcing and Management
Excipient manufacturer adds three tablet binding and disintegration products.

PTSM: Pharmaceutical Technology Sourcing and Management
Manufacturing will be carried out at the Pfizer Newbridge, Ireland, facility, which is now part of Pfizer CentreOne’s contract manufacturing network.

PTSM: Pharmaceutical Technology Sourcing and Management
The agency sent a warning letter to Badrivishal Chemicals & Pharmaceuticals detailing several CGMP violations.

PTSM: Pharmaceutical Technology Sourcing and Management
The agency sent a warning letter to Chongqing Pharma Research Institute Co., Ltd. citing data integrity violations.