PTSM: Pharmaceutical Technology Sourcing and Management
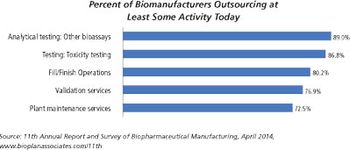
In-house biologics fill/finish operations have invested in new technologies and have available capacity while small-molecule fill-finish facilities work at near-capacity on legacy equipment.

PTSM: Pharmaceutical Technology Sourcing and Management
In-house biologics fill/finish operations have invested in new technologies and have available capacity while small-molecule fill-finish facilities work at near-capacity on legacy equipment.

PTSM: Pharmaceutical Technology Sourcing and Management
Experts review considerations for selecting excipients for solid-dosage drug performance and manufacturability.
PTSM: Pharmaceutical Technology Sourcing and Management
Better barrier materials and software modeling streamline the blister-package design process.
PTSM: Pharmaceutical Technology Sourcing and Management
Custom API manufacturers firms that offer specialized capabilities will benefit from the continuing trend toward pharmaceutical outsourcing.

PTSM: Pharmaceutical Technology Sourcing and Management
Catalent will add potent containment for blending and granulation at its New Jersey manufacturing Center of Excellence.

PTSM: Pharmaceutical Technology Sourcing and Management
PPD announced that it completed an expansion to its Athlone, Ireland facility, resulting in 20,000-square-feet of dedicated space for warehousing and distribution purposes.

PTSM: Pharmaceutical Technology Sourcing and Management
FDA has approved the Ash Steven’s manufacturing facility in Riverview, MI for the manufacture of Amotosalen.

PTSM: Pharmaceutical Technology Sourcing and Management
The new facility will feature dry powder cell-culture media capabilities.

PTSM: Pharmaceutical Technology Sourcing and Management
The new facility will feature new research and development capabilities.

PTSM: Pharmaceutical Technology Sourcing and Management
The acquisition of Aptuit's Glasgow, UK and Indiana, US facilities will add sterile injectable formulation development and expand AMRI's analytical services capabilities.