
With so many personnel involved in the development and manufacture of pharmaceuticals, proper training of all staff is key to ensuring a quality product.

With so many personnel involved in the development and manufacture of pharmaceuticals, proper training of all staff is key to ensuring a quality product.

This paper explores the legal and regulatory framework around 3D drug printing, particularly for personalized medicine, considering regulatory compliance, business concerns, and intellectual property rights.

Centogene NV and ROPAD consortium publish data from a landmark study identifying genetic variants that may respond to innovative cell and gene therapies.

Automation technologies provide real potential to improve responsiveness and decision-making in drug development.
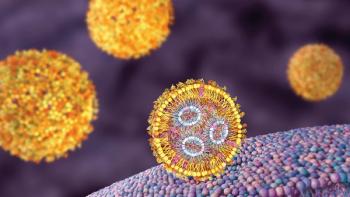
LNPs have gained solid ground as a drug delivery system for mRNA due to their success in the vaccines arena.

A statistical procedure based on a quality range is proposed for demonstrating comparability between pre-change and post-change process slopes in an accelerated or stressed stability study.

As the field of bioanalytical testing evolves, it is important for drug developers to stay at the forefront of the advancements to ensure they remain competitive.

The European Commission has developed a roadmap aimed at reversing the rising trend of cancer across the European Union.

The 340B program is under greater scrutiny with more transparency for drug access being demanded by industry.

Aligned with our audience’s preferences, PTE will be a fully digital publication from 2025 onwards.

All CGMP requirements, including supporting activities, are critical in aseptic sterile manufacturing to ensure product quality and patient safety, says Susan J. Schniepp, distinguished fellow at Regulatory Compliance Associates.

The ROSS Model 42N-10 has a working capacity of 10 cubic feet and is designed for free-flowing materials with bulk densities up to 100 lbs per cubic foot.
Datwyler has launched new coated plungers in its NeoFlex line that are suitable for large volume biologics.