
Expansions

Expansions

Congress Focuses on FDA Inspections of Foreign Drug Facilities; Medical Students Oppose Big Pharma's Influence on Campus; FDA Revises Postmarketing Reporting Requirements

There are at least two major factors that will shape the manufacturing industry as a whole. Daniel Ruppar, Industry Manager (North America) for the Pharmaceuticals and Biotechnology Division of Frost & Sullivan predicts that, due to continued globalization, we can expect to see "growth centers for the industry move to emerging market sectors."
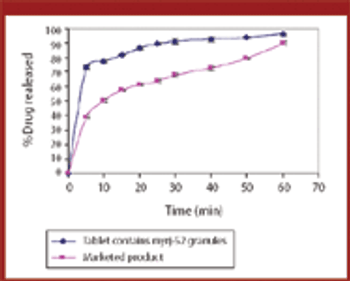
Various manufacturing techniques can improve a drug's solubility, thus increasing its bioavailability. The authors examined whether melt granulation can enhance drug solubility using meloxicam as the drug substance and myrj-52 as the binder.

Contract service providers exist to help clients meet their strategic and financial business objectives. So any prognostication on what the pharmaceutical outsourcing industry will look like in 30 years is really a forecast of the nature and speed of changes in pharmaceutical technologies and business models.

A surge in capacity in contract microbial and mammalian cell-culture is underway to meet rising production needs for biopharmaceuticals.

I foresee dramatic future changes in five distinct areas of the pharmaceutical industry: Research, Regulatory Approval, Production, Sales & Marketing, and IT Infrastructure.
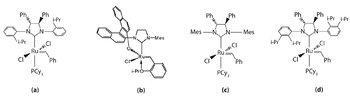
Catalysis plays a critical role in the synthesis of active pharmaceutical ingredients (APIs). It provides a way to improve yield, achieve desired stereoselectivity, improve reaction conditions, and synthesize increasingly complex APIs. Recent advances in catalyzed-olefin metathesis reveal its value and promise in the pharmaceutical industry.

The dynamics of the R&D pipeline could undercut recent CRO and CMO successes.

To keep up with applications, FDA promotes new testing and administrative methods.

In honor of Pharmaceutical Technology's 30th anniversary, the editors conducted a survey of 320 readers last spring to discuss industry advances and future directions. Here are some of your foward-looking responses.
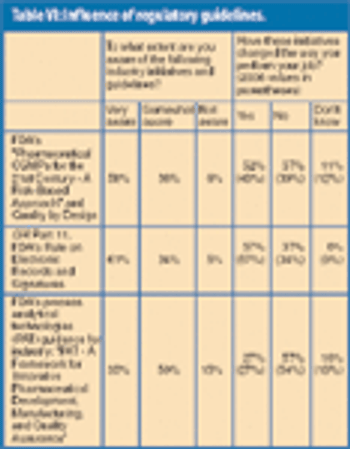
Nearly 1300 pharmaceutical employees provide insights into the issues most relevant to their jobs and the state of the industry workforce today.

Centrifuge processes many materials; New features for quality-management software; Unit simplifies powder discharge

Individualized dosing for specific patient needs has been the goal of medical and pharmacotherapy specialists since they first envisioned pharmacogenetic evaluation. With the measurement of individual levels of metabolism, the optimum dose can be calculated for each individual patient.

As we get ready to head into the new year, chief executive and financial officers may be making their wish lists for 2008. Grow revenue. Increase pipeline. Establish new facility abroad. Acquire small-sized generics company. And so forth. But why not add something "green" to the list?Why not consider ways to help the environment while aiming for success?

Nanotechnology offers an unprecedented opportunity in the rational delivery of drugs and vaccines (1, 2–4).

Industry and regulatory organizations agree that the current focus on product quality will play a major role in shaping pharmaceutical development in the future. Key to this assessment of quality are the methods and technologies in pharmaceutical analytical testing.

Drug makers, drug inspectors, and drug consumers need to demand that new drugs be effective.

How do you answer an inspector's questions? How do you validate analytical methods? One book has answers for compliance professionals.

The "why" things are done in this industry is just as important as the "what"and "how."
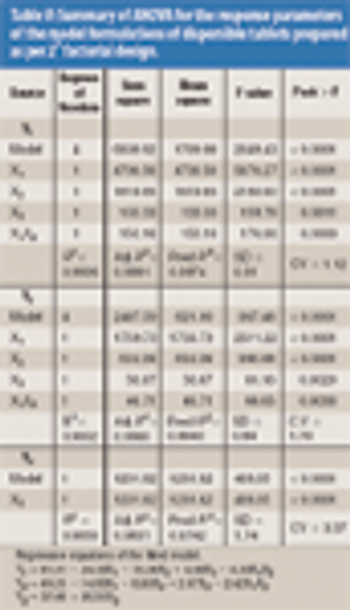
The authors analyzed the effects of complexation as well as the levels of ammonium bicarbonate and crospovidone on tablet wetting time (WT), disintegration time (DT), and percent dissolution efficiency at 60 min (%DE60).

Although the highly regulated pharmaceutical industry tends to be more like the tortoise than the hare when it comes to adopting new ideas, pharmaceutical packaging will be dramatically different 30 years from now in 2037.

"May you live in interesting times," goes the allegedly ancient Chinese curse. Well, these have been "interesting times" indeed for those working in the pharmaceutical industry.