
The authors present a new approach to risk assessment for aseptic processing that emphasizes the contributions of personnel.

The authors present a new approach to risk assessment for aseptic processing that emphasizes the contributions of personnel.

USP monographs, if they are consistently observed and applied, can help reduce medical gas errors.

"It was just after FDA had initiated the Pre-Approval Inspection process," reports our GMP Agent-in-Place.

Oddly for a technical meeting, the Oct. 5–7 AAPS Workshop on Pharmaceutical Quality Assessment focused on words, with an entire session devoted to definitions and countless discussions of meaning and nuance.

As industry moves to real-time feedback control, even conventional syntheses will increasingly resemble living systems.

New leadership must address political and scientific issues as the Medicare drug benefit alters manufacturer incentives.
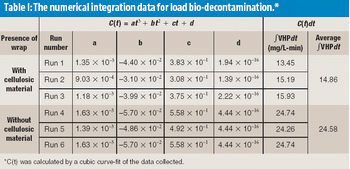
The authors focus on the effects of cellulosic materials during the performance qualification validation of a transfer barrier isolator used for the purpose of sterility testing.

Moheb Nasr, PhD, director of the newly created Office of New Drug Quality Assessment at the US Food and Drug Administration (Rockville, MD, www.fda.gov), has proposed creating a "regulatory agreement" between FDA and sponsors to govern the chemistry, manufacturing, and controls (CMC) sections of new drug applications (NDAs).

The pharmaceutical industry can leverage best practices from the extremely competitive semiconductor market.

As major pharmaceutical companies take initial steps to prune their supplier base and negotiate better pricing, nonpharmaceutical companies that took those measures 10 years ago are moving to next-generation sourcing strategies. In designing those plans, these front-runners are applying two key lessons learned in recent years: supplier consolidation often doesn't go far enough, whereas price cutting can go too far.
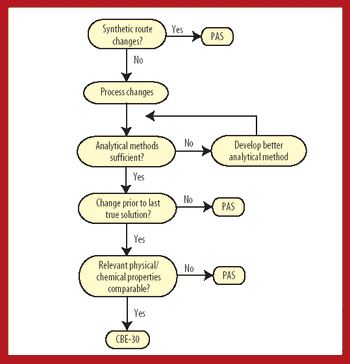
This article provides a PhRMA perspective and recommendations on an FDA guidance currently under development dealing with postapproval changes after the final intermediate of the active pharmaceutical ingredient, i.e., BACPAC II. The concept of a "last true solution" is proposed as an additional point in the assessment of potential risk associated with process changes.
Making active pharmaceutical ingredients (APIs) requires long chains of chemical reactions and large quantities of solvents. Ask API manufacturers how they'd like to improve this process, and the responses are likely to be "make the reactions faster," "make the reactions cheaper," or "make the reactions more efficient." Then after all these economically driven answers, you might here, "make the reactions more environmentally friendly."
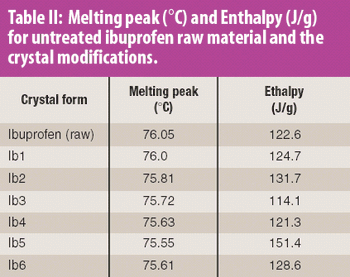
The common crystal form of ibuprofen was changed to optimize processing and manufacturing properties. Six modified crystal forms were prepared and assessed for dissolution, morphology, particle size, density, thermal characteristics, powder x-ray diffractometry, flow properties, and tabletability.