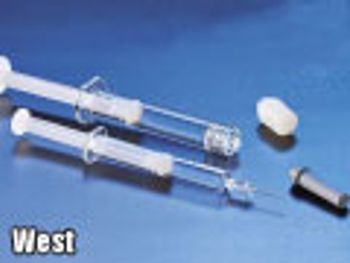
The authors describe the ways in which plastic prefilled syringes can be an alternative that provides consistent performance, protects drugs prone to degradation, and enhances patient safety.

The authors describe the ways in which plastic prefilled syringes can be an alternative that provides consistent performance, protects drugs prone to degradation, and enhances patient safety.

The author describes how Merrion Pharmaceuticals reformulates parenteral drugs into tablets and capsules that are easier for patients to take and provide better bioavailability.

The author details the factors in formulation design, requirements in facilites and equipment, and validation criteria for aseptic formualtions.

A Conversation with Greystone Associates' George Perros. This article contains bonus online-exclusive material.

The authors used a light-transmission-based static division system to detect particles of foreign contaminants in prefilled vials.

The author discusses the risks involved with aseptic processing, methods and tools used to identify and control risk, and regulatory guidelines relevant to the risk-management process.

A Conversation with Otonomy

This article provides an update to the author's Viewpoint column, "Changes to Vial Labels May Affect Patient Safety," which ran in Pharmaceutical Technology's March 2009 issue.