
As the pharmaceutical supply chain expands, sponsor companies need to weigh all their options.

As the pharmaceutical supply chain expands, sponsor companies need to weigh all their options.

Industry is taking a step forward by addressing supply-chain integrity through the formation of Rx-360. PhamTech's podcast explores the subject.
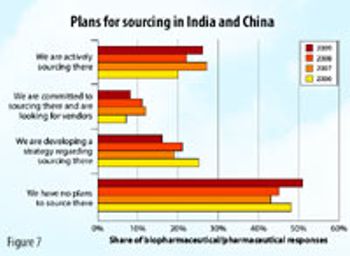
The contract services industry may not be as robust in 2009 as it has been in previous years, but it's not as bad as many people think.

A roundtable with pharma majors Pfizer and Johnson & Johnson, moderated by Jim Miller.
The author provides advice about evaluating contract analytical laboratories and establishing an effective procedure for working with them to perform reliable stability studies.

A Q&A with Pfizer CentreSource, moderated by Jim Miller.

The authors explain waivers and deferrals for pediatric studies of drugs and biologics as provided by the Pediatric Research Equity Act of 2007.

A summary of recent changes to European regulatory requirements for pediatric formulations.

Leading experts share their perspective on the specialized requirments when developing a pediatric formulation and examine dosage forms that can be used for this patient class in this roundtable moderated by Patricia Van Arnum.

Leading contract manufacturing organizations share their views on the current and future market dynamics shaping pharmaceutical outsourcing.