Traditional glass and polymeric materials compete for market share in primary packaging for parenteral drug products.

Traditional glass and polymeric materials compete for market share in primary packaging for parenteral drug products.
The delivery device and drug form should be considered when choosing a test method for identifying and measuring particulates in inhaled drug products.

T&D’s new compact, battery-powered -75wf/nw line of thermocouple data loggers feature a temperature range of -199 to 1760 °C and free cloud storage.

The PowerMix Model PDM-10 from Ross, Charles & Son offers high-shear mixing for hard-to-mix applications with low flowability.

The Analysette 28 ImageSizer from Fritsch provides analysis of particle shape and size for dry measurement of powders and bulk solids and wet measurement of suspensions and emulsions.

The BIOne 1250 bioprocess control station from Distek is a benchtop-scale bioreactor for mammalian and microbial models.

After 30 years of biologic-drug advances, the industry and patients still have a lot to learn.
Biopharma seeks alternatives that meet the needs for next-gen biologic drug production.

With the right excipients, formulators can control when, where, and how an API is released.
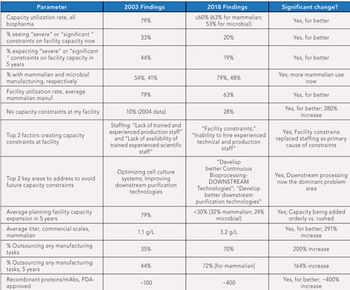
This article highlights 15 years of changes in biopharmaceutical manufacturing.

The author reviews current approaches to sterile containment and compares several sealed transfer and barrier techniques, including isolators, restricted access barrier systems, and split butterfly valve technology.

FDA seeks more efficient testing to spur development of less costly biotech therapies.

EMA, the leading driver behind GMP inspection collaboration schemes, will be cutting back its involvement because of the need to concentrate on its relocation to Amsterdam.

Knowing and addressing regulatory expectations early on can avoid unexpected delays later, says Siegfried Schmitt, principal consultant at PAREXEL.

Pharmaceutical Technology spoke with Sharon Ayd, executive vice-president of Pre-approval Pharmaceuticals and chief scientific officer at Regulatory Compliance Associates, about developing a corrective action and preventive action (CAPA) plan.

Maintaining good quality control practices throughout the entire manufacturing process requires robust development, a drive toward product and process understanding, and pre-established, comprehensive written procedures that are consistently reviewed and updated.

Traditional barriers between upstream and downstream bioprocessing are slowly starting to break down, as biopharma embraces more advanced analytics and process control.
Vendors are offering template-based models and direct data-filing services to help smaller companies meet imminent deadlines.

Macromolecular drugs are typically injected, but oral dosage forms are being developed to improve the treatment of gastrointestinal conditions such as inflammatory bowel disease.

Click the title above to open the Pharmaceutical Technology July 2018 issue in an interactive PDF format.