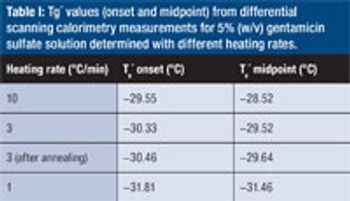
The authors evaluate the thermal properties of gentamicin sulfate as a small-molecule drug model in optimizing the freeze-drying cycle.

The authors evaluate the thermal properties of gentamicin sulfate as a small-molecule drug model in optimizing the freeze-drying cycle.

Peptides and related technologies to are starting to improve production.

Q&A with Peter Smith, Strategic Compliance Consulting, PAREXEL International, and a former senior official with FDA, on change management best practices.
When designing stability protocols, formulation, storage, and dosing conditions must be assessed.

New product reviews for June 2012.

IQ Consortium representatives explore and define common industry approaches and practices for applying GMPs in early development.
Extensive physicochemical characterization of the innovator product and the proposed biosimilar provides the foundation for demonstrating biosimilarity.
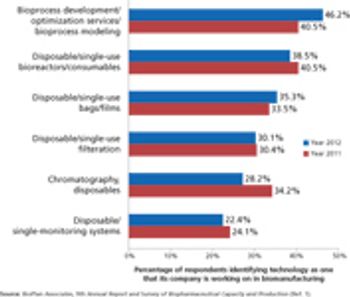
Industry wants more innovation, but can suppliers meet customer needs?

A Q&A with Larry Kadis, President and CEO of Federal Equipment, on recent industry trends.

Highlights included the latest in pharmaceutical packaging equipment, containers, and labels and new capabilities among contract service providers.

BIO is calling for a more patient-centric approach to user-fee reauthorization.

How to avoid invisible and airborne contamination.

US Pharmacopeia documents best supply-chain practices and seeks broad input on proposal.

New price-control policy has domestic and global firms waiting on the sidelines to launch products.

Partnerships between industry and academic medical centers are expanding to meet R&D needs.

New coating technologies achieve high uniformity and reduce waste through mixing system advances and pan and airflow configuration.

UK chooses to use off-label drug indication to cut healthcare costs. Will others follow suit?

New legislation, government programs aim to bolster drug discovery and reduce regulatory hurdles.

Does global development have to entail multiple comparability studies?

Even the slightest of errors in exponential calculations can cause the biggest of headaches.

Looking For Fingerprints