
A book guides readers through the regulatory requirements for computerized quality systems.

A book guides readers through the regulatory requirements for computerized quality systems.

A successful quarantine program requires collaboration among all departments of a pharmaceutical facility.

As regulators work to curb counterfeiting, industry finds benefits to gaining granular data about the supply chain. This article contains bonus online-exclusive material.
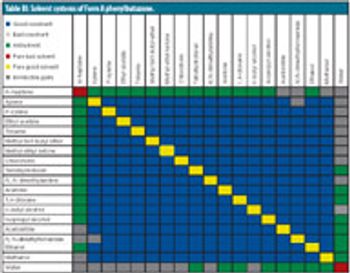
The authors describe the importance of a rapid and an abbreviated screening strategy in initial solvent screening. This article contains bonus online-exclusive material.
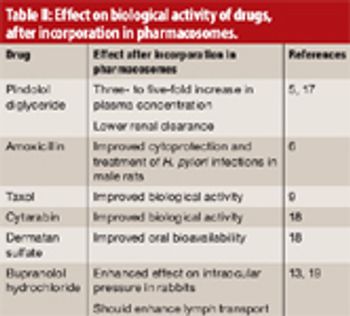
Pharmacosomes can pass through biomembranes efficiently and possess several advantages over traditional vesicular drug-delivery systems.

Compliance features help patients follow medication regimens correctly.

Misleading the public about their investments-be it money or medicine-is unacceptable.

CROs and CMOs expand to gain a piece of the market for clinical trial materials.

Pharma companies that sell redundant facilities could endanger their supply chains.

Follow-on biologics could unleash the potential of several industries and may even spark economic recovery.

It can take a lot of work to make sure nothing happens.

Short-term problems in software or hardware lead to long-term manufacturing troubles.
Debates about science, manufacturing, and European regulations will shape the approval process for follow-on biologics in the United States.

Editors' Picks of Pharmaceutical Science & Technology Innovations
Despite its shrinking domestic economy, Ireland is determined not to let its pharmaceutical industry fade into the shadow of global recession.

Agency officials and manufacturers anticipate stricter enforcement of drug safety and quality.

Brief pharmaceutical news items for June 2009.

FDA leaders explain the purpose and plan for ICH's three quality guidelines.

The Question-Based Review (QbR) initiative of the Office of Generic Drugs has reached its second full year in 2009. Special from the Journal of Validation Technology.

A novel cleanroom apparel design incorporates modern concepts to help minimize contamination. Take a tour of the design.