
Pharmaceutical science and technology news.

Pharmaceutical science and technology news.

Arkema Inc. (Philadelphia, PA, www.kynar.com) has launched the "Kynar Flex" fluoropolymer for use in flexible tubing applications.

Regulation: FDA Steps Up Action Against Counterfeit Drugs, Warns US Consumers Against Fake Medicine from Mexico

A myopic focus on short-term growth can endanger the community. The question is, can we enlighten our self-interest before the edifice falls down around our ears?

The Chapter 11 bankruptcy filing of aaiPharma (Wilmington, NC, www.aaipharma.com), parent of AAI Development Services, was the culmination of an agonizing year-long odyssey. It also was a first step in restoring one of the best-known brands in the CRO industry.

Officials at the US Food and Drug Administration are working with industry and academia to develop more efficient and reliable drug production processes that can ensure a consistent supply of high-quality therapies. A modern manufacturing system based on harmonized regulatory policies across global regions is critical for meeting public demand for safe and effective medicines, while also reducing production costs and eliminating waste.

Once considered mainly an afterthought in a company's lifecycle-management strategy, controlled-release dosage forms are now positioned at the forefront of many formulation strategies. In contrast to drug discovery, formulation work focuses not only on the intricacies of the active pharmaceutical ingredient (API), but also on fine-tuning the excipients, the release profile, and the delivery mechanism to provide optimal therapeutic benefit. Because of their wide range of applications and functionalities, especially in controlled-release therapies, polymers are among the most widely used excipients.

In his Feb. 2005 viewpoint article, "In Defense of Singlet Testing," Torbeck (1) draws an important philosophical distinction between "standards" and "specifications." He argues that specifications are criteria selected by manufacturers for statistical control of their products, whereas compendial standards are absolute requirements. This distinction is entirely compatible with modern concepts of statistical process control.
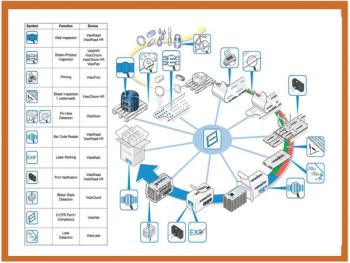
Most experts recommend layering protective technologies by selecting a combination of overt and covert techniques.

Wet granulation is a size-enlargement process in which a liquid is used to achieve the agglomeration of solid particles. Agglomeration improves particles' tableting properties by rendering them free-flowing, nonsegregating, and suitable for compression (1).