
Applications of ZFN technology in biopharmaceutical cell-line engineering.

Applications of ZFN technology in biopharmaceutical cell-line engineering.

Compositing samples is appropriate under certain circumstances but raises caveats on how and when it should be applied.

Manufacturers work with international authorities to harmonize drug registration and supply-chain oversight.

James Stumpff, Principal Consultant at PAREXEL discusses supplier management and FDA's expectations.

While there are those who want combination products to be controlled by a centralized pharmaceutical-type approval system, the majority of the medical technology industry wants to retain a decentralized device-focused approach.

The rejection by India's Supreme Court on Novartis' Glivec/Gleevec (imatinib mesylate) and other recent case law raise important issues on patent strategies for solid forms.
The impact of new delivery technologies in designing peptide therapies.

Solid-state chemistry is an important part of drug development, and public research is advancing the field.

A Q&A with Stuart E. Needleman, President and Chief Operating Officer of Aptuit, on recent industry trends.

New US patent rules change the playing field for open innovation.

Prior to price escalation of pharmaceutical products in Brazil, the country's regulatory authority released a study on price-cap control and its benefits in the past years.

The author examines dry dispersion and outlines the related analytical method development.

The author suggests co-opetition as a future model for collaboration in drug development.

Lyophilization technologies for controlled nucleation.

Cocrystals are used to improve the performance of APIs that have non-ideal physiochemical properties by cocrystallizing the API with a second compound that modulates the API to provide a way to improve a drug's bioavailability, stability, and processability.

New product reviews for May 2013, featuring automation, IT, and process control systems.

FDA faces budget crunch; Supreme Court hears key cases

Pharmaceutical Technology spoke with INTERPHEX 2013 conference-session presenters to gain insight on trends in facility and process design.

Optimized freeze-drying cycles can offer scientific and business advantages.
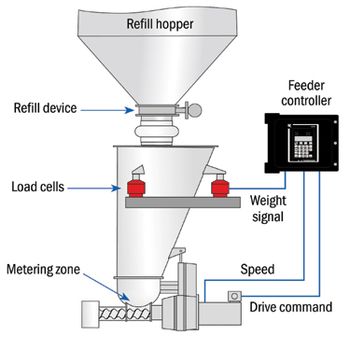
Loss-in-weight feeders provide high accuracy for batch or continuous processes.

Outsourcing is weighing in more as a tactic for cost-cutting, but it is still not the primary weapon.

The author provides a review of FDA's guidance document, Guidance for Industry: Q11 Development and Manufacture of Drug Substances, and its relation to the International Conference on Harmonization's Q11 document and its application to the industry.

Wanted: Article contributions on drug and process development topics.

Click the title above to open the Pharmaceutical Technology May 2013 issue in an interactive PDF format.