
Strong growth in biopharmaceuticals bodes well for contract manufacturing, but the perils and the promises of pipelines remain.

Strong growth in biopharmaceuticals bodes well for contract manufacturing, but the perils and the promises of pipelines remain.

A major CRO ventures further into proprietary drug development.

The president of BIO proposes the ingredients needed for industry growth.

Process steps, GMP documents, a purification vessel, and validation seem to disappear.
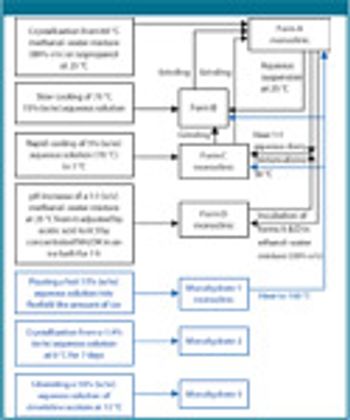
The authors describe the importance of a rapid and an abbreviated screening strategy by initial solvent screening in 20-mL scintillation vials.

The Japanese government is eager to jumpstart its generic-drug market, but changes must come first.

Taiwan sees to bring its intellectual property laws and enforcment into better alignment with international standards.
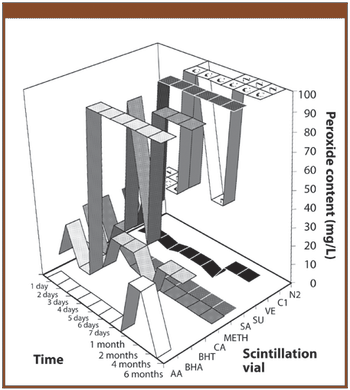
The authors investigate whether the addition of an antioxidant could be used to stabilize the solvent ethyl lactate by preventing the formation of peroxides

The GDP committee of IPEC–Europe is trying to seal one more broken link in the supply chain. This article contains bonus online-exclusive material.

Brief pharmaceutical news items for May 2009.
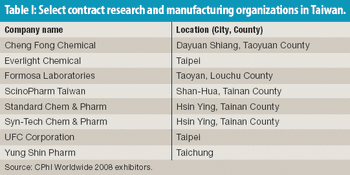
Taking a cue from its electronics industry, Taiwan is seeking to put its biotechnology and pharmaceutical industries on the map. An interactive map shows pharmaceutical activity in Taiwan.

Government funding is slated to boost comparative studies of prescription drugs.

Manufacturers of therapeutic monoclonal antibodies consider new paradigms in purification technologies.

Products at INTERPHEX focused on protection, compliance, and deterring counterfeiting. This article contains bonus online-exclusive material.

The organizations' presidents discuss market exclusivity, approval processes, and pending legislation.

Technology can solve enterprise-level problems.

Editors' Picks of Pharmaceutical Science & Technology Innovations