
Bringing Exubera to market requires extensive collaboration by Pfizer, Nektar Therapeutics, West Pharmaceutical Services Tech Group, and Bespak.

Bringing Exubera to market requires extensive collaboration by Pfizer, Nektar Therapeutics, West Pharmaceutical Services Tech Group, and Bespak.

Buyers strategically target chemistry, manufacturing, and controls development services.

China's State Food and Drug Administration prepares to strengthen the enforcement of good manufacturing practices.

Animal testing and accounting can both be hazardous.

With single-enantiomer separations dominating the blockbuster charts, simulated moving bed chromatography and other multicolumn continuous chromatographic processes offer a quick route to clinical trial materials, along with the resolution, economy, and scalability to support tons-per-year production.
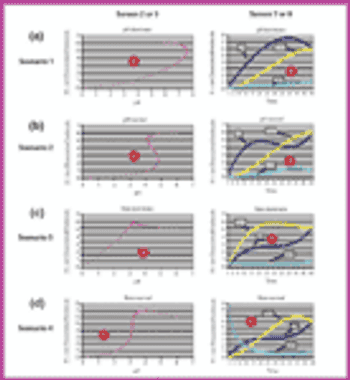
Using Bezier curves, an experimental process controller has been developed for biosynthesis applications in which the inactivity of a pH-sensitive enzyme must be decreased. By taking into account various control scenarios of pH and growth rate, as well as the physical and chemical characteristics of the environment, a suitable human-machine interface can be developed.

At this year's BIO conference, US Health and Human Services Secretary Mike Leavitt predicted that over the coming decade, "Medicine will be transformed from an instinctive art of alleviating symptoms to a science of personalized healthcare." Is industry ready?
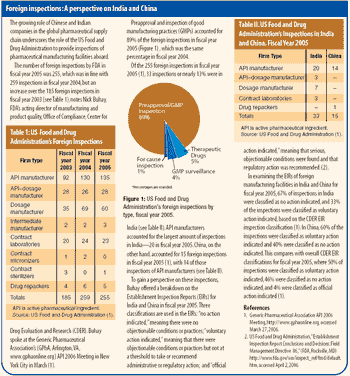
Indian suppliers of active pharmaceutical ingredients and dosage formulations expand in India, the United States, and Europe.

From the most frenetic conference season in recent memory, trying to distill perspective from mental snapshots of Interphex, Wisconsin, and BIO 2006.

Gottlieb challenges industry. First GenerationNext Awards. Implementing PAT. Optimizing site risk. RFID in the supply chain. Biodegradable polyketals. Global drug-market growth moderates.

Manufacturing and formulation innovation spurs drug develop-ment, but raises new safety and quality issues.