
Navigating the Equipment and Machinery Market

Navigating the Equipment and Machinery Market

Pharmaceutical Technology's annual survey on equipment and machinery reveals the spending levels and type of spending made in 2011 and planned for 2012.
In Part III of a three-part article, the authors examine various degradation routes of APIs, impurities arising from interactions in formulations, metabolite impurities, various analytical methods to measure impuritie, and ways to control impurities.

Critical issues that should be considered when scaling up a hot-melt extrusion process.

New product reviews for April 2012.

Collaboration can begin with a conversation.

With financing constrained, biotechnology firms must find ways to sustain innovation.

Recovery audits and other past practices in procurement can improve the bottom line.

Contract API manufacturers and fine-chemical producers roll out capacity and service expansions.

How to use geographic diversification and legacy technology transfers to avoid product shortages.

Visitors will see many packaging innovations at the annual industry exhibition.

Failure to disclose info may work sometimes, but eventually every question will be answered.

The importance of new drug trials to patients, the economy, and science.

Understanding the differences between convenience, target, and self-selected samples.
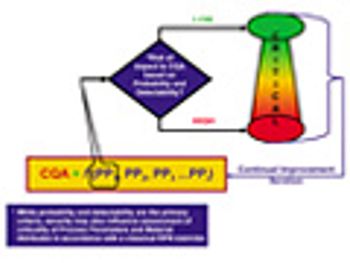
The authors provide an overview of the new ISPE Guide Series on Product Quality Lifecycle Implementation and how the guides can be used in a complementary way with existing guidance from FDA and the International Conference on Harmonization.
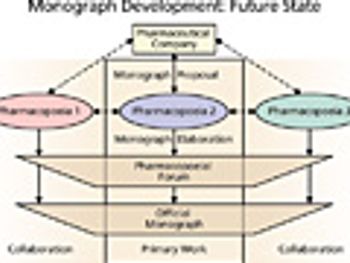
The authors discuss a new approach to address globally harmonized compendial standards.

A Q&A with Erik van den Berg, CEO of AM-Pharma, on recent industry trends.

Experts discuss the best practices for developing a QbD-based lyophilization process.

Soaring opioid use creates challenges for new drug development and supply-chain control.

Comparison of the top GMP deficiencies cited by the PIC/S Participating Authorities.

The confluence of science, technology, and regulation can provide path forward.

Excipient manufacturers expand production capacity and partner to broaden their offerings.

China's drug-distribution network has been a mess for years, but government reforms and industry focus are unveiling new opportunities for market order and growth.

The contract provider needs to know as much as the NDA holder.

The authors detail the possible consequences of noncompliance and a lack of quality control.
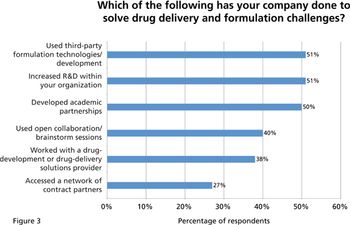
A recent survey examines the industry's views on the chief challenges and technologies in drug delivery and formulation development.

Q&A with Peter Smith and David Elder, Strategic Compliance Consulting, PAREXEL International, on acceptable deviation investigations. Smith and Elder are both former senior officials with FDA.