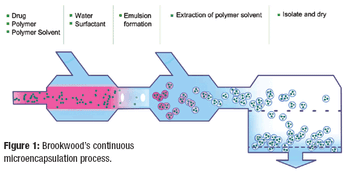
Forty years ago, investors and business speculators shuddered at the prospect of working with India's pharmaceutical industry. The industry was plagued by archaic patent laws and insufficient infrastructure, and only multinational companies (MNCs) were able to exploit its crude resources and monopolistic legal framework. Struggling in the shadows of these MNCs were the Indian pharmaceutical entrepreneurs and a handful of producers. Because more than 70% of the pharmaceutical market value was in the hands of MNCs, India's indigenous pharmaceutical industry was floundering. Its amount of exports was negligible, and the domestic market outlook was bleak because of onerous government regulation.