
Contract manufacturers expand capabilities in aseptic processing, clinical-trial materials supply, and cytotoxic manufacturing.

Contract manufacturers expand capabilities in aseptic processing, clinical-trial materials supply, and cytotoxic manufacturing.
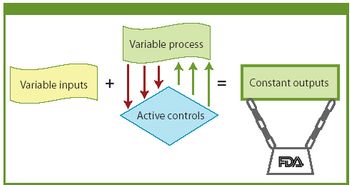
FDA is modernizing and streamlining current good manufacturing practices. The author examines FDA's evolving approach to quality systems and how a manufacturer can implement a quality system framework.

The rise in overseas manufacturing undermines FDA oversight of drug quality.

PharmTech's polls feature user feedback on issues facing the pharmaceutical industry.

Another Asian services provider demonstrates the global nature of pharmaceutical outsourcing.

Brief pharmaceutical news items for February 2008.

USP 467 Residual Solvents will take effect on July 1, 2008. But does the industry understand these specifications-and is it prepared?

A news roundup for February 2008.

The author suggests a route for nanotechnology's future in the pharmaceutical industry.

Can an overload of patent applications lead to the US' demise as a scientific leader?

An updated book summarizes recent research for formulators and drug-delivery specialists.

If at first the product fails, then inspect, inspect again

Jacketed hose provides cleanability; Stopper prevents product waste; Accessories control temperature precisely

A wave of pharmaceutical expansions is expected in Europe this year, surprisingly by Indian companies.

Text sirtuins are the new kinases, according to a presentation given last month at the JP Healthcare Investors Conference in San Francisco by Sirtis CEO Christoph Westphal.