
The injunction blocks rules that required secondary wholesalers to supply pedigrees tracing the chain of custody to the maker.

The injunction blocks rules that required secondary wholesalers to supply pedigrees tracing the chain of custody to the maker.

As technology for impurity analysis improves, scientists are gaining better information and asking for more regulatory guidance.

"Quality by design" (QbD) and "quality risk management" at long last seem to be moving from the buzzword stage to becoming important influences on drug development and manufacturing. A series of quality standards issued by the International Conference on Harmonization (ICH) is encouraging the adoption of common quality-based drug manufacturing approaches designed to reach the "desired state" of drug manufacturing (i.e., more efficient, agile, flexible operations that can reliably produce high-quality drug products with less regulatory oversight). These developments reflect increased pressure to make pharmaceutical manufacturing more efficient and less wasteful and to encourage regulators in all regions to focus on the most critical issues affecting product quality and patient safety.

We, the people who make drugs, are extraordinarily good at what we do.

The role of micro-biological testing in real-time release is too important to ignore.
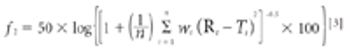
The objectives of this study were to prepare and characterize inclusion complexes of lovastatin with hydroxypropyl-β-cyclodextrin (HPβ-CD) and to study the effect of the complexes on the dissolution rate of lovastatin (LVS). The findings suggest that LVS's poor dissolution profile can be overcome by preparing its inclusion complex with HPβ-CD.

It's what's on the outside that counts, too.

When it comes to ethics, the adage "hindsight is 20/20" is especially applicable. Countless medical and psychological experiments-such as the 1932 Tuskegee syphilis study or Zimbardo's 1972 Stanford mock-prison experiment-were conducted in the name of science and are now plainly recognized as enormous violations of ethical and human rights.
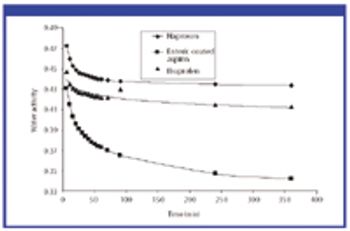
For solid oral-dosage forms, water testing usually is performed to control the chemical, physical, or microbiological properties of the drug product. Measurements of total water as made with Karl Fischer (KF) techniques is not needed and water-activity often will provide a better correlation with changes in chemical, physical, or microbiological properties than KF techniques. In these cases, water activity testing can easily replace KF testing. Water-activity measurements are nondestructive, require little labor, and the equipment required is generally inexpensive. Only a few simple checks are needed to ensure the validity of measurements. Strategies for implementing water activity testing are described.

As custom manufacturers gather for InformexUSA this month, strategies in asymmetric synthesis and catalysis prevail.

Sixty deals were completed in 2006, with a total value of $2 billion.