Pharmaceutical Technology Europe
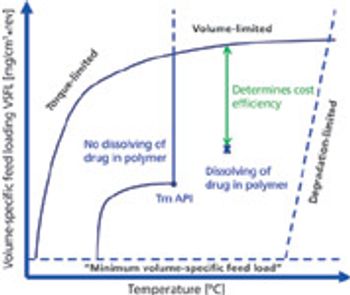
The advantages and disadvantages of hot-melt extrusion in solid dispersion formulations.

Pharmaceutical Technology Europe
The advantages and disadvantages of hot-melt extrusion in solid dispersion formulations.
Pharmaceutical Technology Europe
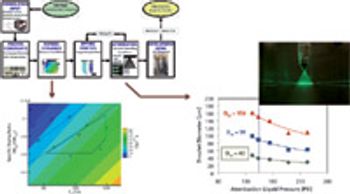
Spray drying is a key process for manufacturing amorphous dispersions because of its breadth of applicability. The wide range of potential atomization techniques and controllable drying kinetics enables amorphous spray-dried dispersions (SDDs) to be produced from a wide variety of active pharmaceutical ingredients (APIs).
Pharmaceutical Technology Europe
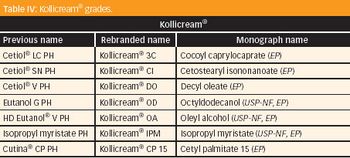
Solubilizers play an important role in dissolving poorly soluble molecules. As the number of poorly soluble lipophilic and/or hydrophobic molecules increases-whether as "brick dusts" or waxy substances-the industry is struggling to identify the appropriate lipophilic excipients (surfactants, solubilizers, solvents or polymers) that can be used to develop such poorly soluble formulations into solid dosages and other forms of pharmaceutical products.

Pharmaceutical Technology Europe
In drug development, the majority of new chemical entities are lipophilic and/or poorly soluble. New actives often fail during development due to these challenging properties.

Pharmaceutical Technology Europe
A Q&A with Karl Kolter, PhD, Head of Pharmaceutical Excipients R&D, BASF

Pharmaceutical Technology Europe
A round table with Catalent and BASF on their new collaboration to provide bioavailability solutions.
Pharmaceutical Technology Europe
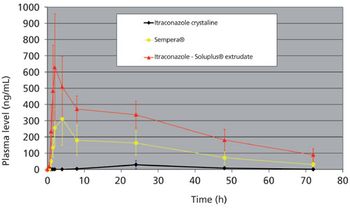
Soluplus®, a graft copolymer of PEG comprising polyvinyl acetate and polyvinyl caprolactam, was specially designed to solubilize poorly water-soluble drugs.

Pharmaceutical Technology Europe
Development of viable dosage forms for poorly water-soluble compounds continues to be a significant challenge for formulation scientists, and insufficient bioavailability of such compounds may result in development delays or failures.