Pharmaceutical Technology Europe
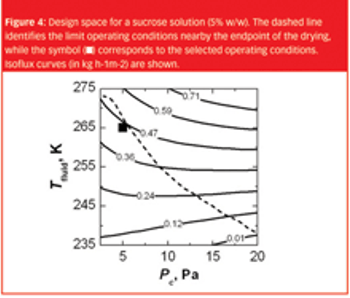
The same recipe obtained in laboratory-scale equipment cannot, without modifications, generally be used to freeze-dry the product in a pilot-scale or industrial-scale freeze-dryer.

Pharmaceutical Technology Europe
The same recipe obtained in laboratory-scale equipment cannot, without modifications, generally be used to freeze-dry the product in a pilot-scale or industrial-scale freeze-dryer.
Pharmaceutical Technology Europe
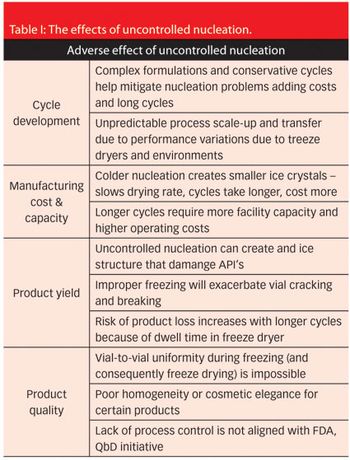
Ideally, all product formulations should experience the same conditions when it comes to lyophilisation, but in practice, however, nucleation can occur.

Pharmaceutical Technology Europe
The increase in aseptic processing driven by the growing number of biologically-derived products has led to an increase in freeze-drying applications in this area at both research and production scales.

Pharmaceutical Technology Europe
Switzerland is an important power in Europe's pharmaceutical industry.

Pharmaceutical Technology Europe
Product degradation is often overlooked compared with other supply chain issues, but if left unchecked can lead to the market entry of substandard medicines, whether through ignorance or gross negligence.

Pharmaceutical Technology Europe
The coming years will show a slowing growth rate in the global spending of medicines, particularly when it comes to branded products.

Pharmaceutical Technology Europe
Lyophilisation or freeze-drying is widely used in the pharmaceutical industry for a variety of reasons.

Pharmaceutical Technology Europe
One of the perks of my job is that I can travel to previously unvisited cities. This month, I was lucky enough to be in Switzerland.

Pharmaceutical Technology Europe
Single-use filtration and fill–finish technologies can be used as part of a lean manufacturing strategy to boost production, and reduce manufacturing waste and costs.